 Author
Author  Correspondence author
Correspondence author
Maize Genomics and Genetics, 2025, Vol. 16, No. 4 doi: 10.5376/mgg.2025.16.0018
Received: 05 Jun., 2025 Accepted: 20 Jul., 2025 Published: 10 Aug., 2025
Wang J.M., Zhang X., and Wang G.F., 2025, Transcriptomic analysis of heat-responsive genes in maize seedlings, Maize Genomics and Genetics, 16(4): 202-218 (doi: 10.5376/mgg.2025.16.0018)
High temperature stress is an important limiting factor affecting the yield and quality of maize (Zea mays). In recent years, with global warming, the problem of reduced corn production caused by high temperatures has become increasingly prominent. Therefore, it is of great significance to analyze the molecular mechanism by which corn seedlings respond to high-temperature stress. This study reviews the physiological effects of high temperature on the growth and development of corn seedlings, as well as the latest progress in exploring high-temperature response genes in corn using transcriptomics technology. It elaborates on the interference of high-temperature stress on physiological processes such as photosynthesis and respiration in corn, as well as the response characteristics such as reactive oxygen species accumulation, antioxidant defense, and hormone level changes. Analyze the expression patterns and protective effects of the corn heat shock protein family genes, the regulatory functions of heat response transcription factors (such as HSF, bZIP, NAC, etc.), and the potential regulatory roles of non-coding Rnas such as miRNA, and summarize the main pathways of high-temperature signal perception and conduction. Especially the role of signaling pathways such as Ca2+, ABA, and MAPK in the heat stress response of maize, and the key regulatory modules and candidate genes are revealed through the gene co-expression network. The preliminary molecular map of the high-temperature response of corn has been constructed, but there are still weak links in the research. This study looks forward to the future and requires the integration of multi-omics techniques to deeply reveal the mechanism of corn heat tolerance, in order to promote the breeding of new corn varieties with stable yields under high-temperature conditions.
1 Introduction
Corn is a crop that is extremely sensitive to temperature, and it may be adversely affected by high-temperature heat damage at all stages of its growth. When the environmental temperature exceeds the optimal temperature range for corn growth (generally 25 ℃-33 ℃ during the day), the growth rate of seedlings decreases, photosynthesis is hindered, and respiratory loss intensifies, resulting in a reduction in the accumulation of plant biomass. Under continuous high-temperature stress, the growth of corn stems and leaves is inhibited, the leaves develop poorly, and premature senescence symptoms occur. In severe cases, the seedlings may wilt or even die. Especially, high temperatures are often accompanied by drought, which further aggravates the water deficiency and insufficient supply of photosynthetic products in corn, and has a more significant impact on seedlings. Studies show that high-temperature heat damage can cause a reduction in corn production of more than 10%, and in extreme cases, for every 1 ℃ increase, it may lead to an additional 7% decrease in corn yield (Kim and Lee, 2023). Therefore, high-temperature stress has become one of the important environmental factors restricting the increase of corn yield and requires sufficient attention.
To mitigate the adverse effects of high temperatures on corn production, breeders are working on developing heat-resistant corn varieties. However, traditional breeding methods are inefficient and there is insufficient understanding of the genetic basis of corn heat tolerance. In recent years, with the development of molecular biology and genomics, people have begun to reveal the mechanism by which corn responds to high-temperature stress at the molecular level and apply it to breeding practice. For instance, through the evaluation of the heat tolerance of large-scale corn germplasm, a batch of high-temperature resistant inbred lines and hybrid materials were screened out, providing a parental basis for heat-resistant breeding. Meanwhile, genetic engineering and gene editing techniques also provide possible approaches for precisely improving the heat tolerance traits of corn (Razzaq et al., 2021; Pandey et al., 2024). In particular, omics technologies such as transcriptomics can identify key genes and regulatory elements in response to high temperatures in high throughput, accelerating the development of heat-resistant related molecular markers and the cloning of candidate genes. The achievements of research on the molecular mechanism of heat response have demonstrated application potential in corn breeding: for instance, quantitative trait loci (QTLS) and associated markers for identifying heat tolerance in corn can be used in heat-resistant molecular breeding. The cloned heat-regulating genes can be used for transgenic methods to enhance the stress resistance of crops. It can be foreseen that an in-depth analysis of the signal transduction and gene regulatory network of corn in response to high-temperature stress will provide important genetic resources and theoretical guidance for the breeding of new heat-tolerant corn varieties.
This study focuses on the response mechanism of corn seedlings to high-temperature stress. Based on the latest research progress, it systematically reviews the physiological responses, transcriptional regulatory characteristics, and molecular network mechanisms of corn under high-temperature stress. This study first introduces the adverse effects of high temperature on the growth and development of corn seedlings and the stress response, laying a foundation for understanding the subsequent molecular mechanisms. Then, it focuses on summarizing the expression characteristics of genes related to corn heat response, including the role of the heat shock protein (HSP) family, heat response transcription factors, and non-coding Rnas, and elaborates on the pathways and molecular networks by which corn perceives and transmits high-temperature signals. Special attention was paid to typical signaling pathways such as Ca2+, abolic acid (ABA), and mitogen-activated protein kinase (MAPK). Then, the differences in gene expression in different tissues and at different times were compared to analyze the tissue specificity and temporal dynamic patterns of heat stress responses in maize. On this basis, summarize the potential candidate genes and their regulatory patterns identified through transcriptome analysis, and explore the application prospects of these genes in heat-tolerant breeding. This study aims to construct a molecular map of corn's high-temperature response, deepen the understanding of the heat tolerance mechanism of corn, provide a scientific basis for improving the heat tolerance traits of corn, and also offer reference value for the heat resistance research of other crops.
2 Physiological Responses of Maize Seedlings to Heat Stress
2.1 Disruption of photosynthesis and respiration caused by high temperatures
The most direct impact of high-temperature environments on corn seedlings is manifested in the decline of photosynthetic efficiency and the disorder of respiratory metabolism. Under suitable temperatures, corn synthesizes a large amount of assimilates through photosynthesis for growth and development. However, when the temperature suddenly rises above 35 ℃, the stomata on the leaves close excessively to reduce water loss, resulting in a significant decrease in the rate of carbon dioxide assimilation. Research has found that high-temperature stress can damage the structure and function of photosystem II (PSII), reduce the photochemical quantum yield, and cause adverse changes in chlorophyll fluorescence parameters (Doğru, 2021). Meanwhile, high temperatures accelerate the rate of corn respiration, causing excessive consumption of a large amount of carbohydrates and generating excessive free radicals and other by-products, which further inhibit the photosynthesis process. For instance, when comparing two corn varieties with different heat tolerations, it was found that under high-temperature treatment, the net photosynthetic rate (Pn) and chlorophyll content of the sensitive variety decreased much more than those of the heat-tolerant variety, while the intensity of respiratory oxygen release abnormally increased (Wang et al., 2024). High temperatures also cause changes in leaf structure: leaves become thinner and the density of stomata decreases, thereby reducing photosynthetic capacity. High temperatures interfere with the photosynthetic electron transfer and carbon assimilation processes of corn seedlings by affecting stomatal behavior and the photosynthetic membrane system, and also enhance respiratory decomposition. Together, these two factors lead to insufficient accumulation of photosynthetic products and hinder plant growth.
2.2 Accumulation of reactive oxygen species (ROS) and activation of antioxidant defense systems
High temperature stress often leads to excessive accumulation of reactive oxygen species (ROS) in corn seedlings, putting the cells in an oxidative stress state. High temperatures can disrupt the normal function of the electron transport chain in organelles such as chloroplasts and mitochondria, leading to incomplete oxygen reduction and the generation of ROS such as peroxides and superoxide anions. An appropriate amount of ROS can act as a signaling molecule to trigger defense responses, but excessive ROS can cause membrane lipid peroxidation, enzyme inactivation and DNA damage. The ROS content of corn seedlings significantly increased at high temperatures, such as the levels of hydrogen peroxide (H2O2) and superoxide anion (O2-), which rose markedly. The accumulation rate was relatively low in heat-resistant materials, but increased more rapidly in sensitive materials. To resist oxidative damage, corn activates the body's antioxidant defense system, including both enzymatic and non-enzymatic pathways. The enzymatic antioxidant system mainly includes superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), and ascorbic acid peroxidase (APX), etc. The activities of these enzymes are rapidly upregulated at the initial stage of high temperature to eliminate excessive ROS. For example, the activities of SOD and POD in heat-tolerant varieties increased by more than 30% compared with the control after high-temperature stress, while the increase in the related enzyme activities of sensitive varieties was small or even decreased (Cao et al., 2021). Non-enzymatic antioxidants such as ascorbic acid, glutathione, proline and flavonoids also accumulate under high-temperature stress. They can directly eliminate free radicals and stabilize the membrane system structure. However, when the high temperature is too intense or lasts for too long, the antioxidant system may find it difficult to fully counteract the damage caused by ROS, resulting in a significant increase in the content of malondialdehyde (MDA), a lipid peroxidation product of the cell membrane, and an increase in the relative permeability of the cytoplasmic membrane. This is particularly evident in high-temperature sensitive corn materials: experiments observed that the MDA content in sensitive inbred line seedlings increased by more than twice after high-temperature treatment compared with the control, causing severe damage to membrane integrity, while the increase in MDA in heat-tolerant inbred lines was relatively small (Huo et al., 2023). High temperatures cause a large accumulation of ROS and induce the antioxidant defense system of corn to respond. The dynamic balance between the two determines the degree of oxidative damage suffered by the plants and is one of the important factors affecting the heat tolerance of corn.
2.3 Hormonal metabolism changes in response to heat stress
Plant hormones play a key regulatory role in the response of corn to high-temperature stress. High temperatures can disrupt the hormonal balance within corn, causing changes in various hormone levels and metabolic pathways. Among them, abscisic acid (ABA) is regarded as an important response hormone under high-temperature stress. Studies have shown that high temperatures can induce a rapid increase in endogenous ABA content in corn seedlings, while growth-promoting hormones such as cytokinin (CTK) and auxin (IAA) relatively decrease. The accumulation of ABA helps to close stomata and reduce transpiration, thereby enhancing the plant's stress resistance, but it may also inhibit growth. As Cheikh and Jones' early research found, under high-temperature conditions, the ABA content in corn plants increases and the development of young corn panicles is hindered. Applying 6-benzyladenine (an artificial cytotin) to corn can partially counteract the negative effects of ABA and promote normal grain filling. In addition, calcium ions (Ca2+) and ABA work in synergy to play a role in heat signal transduction: exogenous application of both Ca2+ and ABA can enhance the activity of antioxidant enzymes in corn seedlings, reduce the level of membrane lipid peroxidation, and thereby improve heat tolerance. In addition to ABA, the roles of other hormones such as gibberellin (GA), salicylic acid (SA), jasmonic acid (JA), and brassinolide (BR) in the heat stress response of maize have also been reported. GA promotes the growth of corn at normal temperature, but at high temperatures, overly strong GA signals may intensify the contradiction between growth and stress resistance. On the contrary, moderately reducing the GA signal can decrease growth consumption and is beneficial for heat tolerance. Recent combined transcriptomic and metabolomic analyses have found that high-temperature stress significantly affects the expression levels of auxin and abolic acid related metabolic genes in corn seedlings. For instance, auxin polar transport proteins and signaling elements are suppressed at high temperatures, while the NCED gene, a key enzyme in ABA synthesis, is upreregulated, indicating that corn activates stress resistance by increasing the ABA/IAA ratio. It is worth mentioning that the transcription factor ZmGBF1 has been confirmed to directly bind and regulate the ZmxCXE2 gene of maize carboxylesterase, thereby increasing the metabolic level of gibberellin and enhancing the heat tolerance of plants. Cao et al (2021) revealed the close connection between hormonal pathways and transcriptional regulation in the heat stress response of maize. High-temperature stress regulates the metabolism and signal transduction of various plant hormones such as ABA, triggering a series of downstream responses, thereby guiding resource reallocation, inhibiting growth and activating stress protection mechanisms in corn seedlings.
3 Expression Characteristics of Heat-Responsive Genes
3.1 Expression patterns and protective roles of heat shock protein (HSP) families
Heat Shock Protein (HSP) is a type of molecular chaperone protein that is abundantly expressed in plants under adverse conditions such as high temperature. It is widely present in crops such as corn and plays an important protective role in heat tolerance. According to molecular weight, the HSP family is classified into subcategories such as HSP100, HSP90, HSP70, HSP60, and small molecule HSP (sHSP). Among them, small-molecular-weight HSPS (such as HSP17.9, HSP18, etc.) accumulate most significantly under high-temperature induction. Transcriptome analysis indicates that high-temperature stress can cause a strong upregulation of a large number of HSP genes in corn: for instance, after the B73 seedlings of the corn inbred line were treated at 45 ℃ for one hour, the transcriptional levels of as many as dozens of HSP genes increased several times or more. Especially the HSP70 family and the small HSP genes of HSP17-30kDa are extremely sensitive to temperature changes. Their promoters often contain thermal shock elements (HSE), which can be rapidly activated by the binding of thermal shock transcription factors. The high expression of these HSPS at high temperatures serves to act as molecular chaperones, facilitating the refolding, assembly and transport of damaged proteins within cells, preventing the aggregation of non-natural conformational proteins and thereby reducing cytotoxicity. For instance, small HSPS such as ZmHSP16.9 and ZmHSP18 in corn accumulate in large quantities under heat stress, which can effectively bind to denatured proteins and maintain some of their structures. Once the stress is relieved, they assist in their reconstitution. HSP70 and HSP90 are mainly located in the cytoplasm and organelles, helping to stabilize various functional protein complexes. A study on the HSP90 gene family in corn identified nine ZmHSP90 genes and found that they exhibited tissue-specific induced expression patterns under different heat stress conditions and were involved in protein folding and the assembly of signal complexes. As a member of the HSP100 family, HSP101 also plays a significant role in the heat-sensitive process of meiosis in pollen mother cells. Li et al. (2022) reported that ZmHSP101 is highly expressed during the formation of corn microspores and helps maintain the protein homeostasis of pollen mother cells. The loss of its function can lead to male sterility at high temperatures. These pieces of evidence indicate that different types of HSPS play their respective roles in the heat tolerance of corn: small HSPS are responsible for the immediate "emergency rescue" of damaged proteins, HSP70/HSP90 and others maintain normal cell metabolism and the operation of signaling pathways, and HSP100 is involved in heat memory and long-term adaptation. Therefore, the high expression pattern of HSP family genes is a typical "fingerprint" feature of corn in response to high-temperature stress, and its products endow plant cells with heat protection at the cellular level through molecular chaperone mechanisms.
3.2 Regulatory functions of heat-responsive transcription factor families (HSF, bZIP, NAC, etc.)
Transcription factors are crucial regulatory elements in the process of heat stress signal transmission. A series of studies have identified multiple families of transcription factors involved in the thermal response of corn, including thermal shock transcription factor (HSF), basic leucine zipper protein (bZIP), NAC, AP2/ERF, WRKY, etc. Among them, the HSF family is a central regulator that directly senses protein denaturation signals and triggers the expression of downstream defense genes. The maize genome contains over 25 HSF genes, and their expression profiles under high-temperature stress are complex: most HSFS are rapidly upregulated and activated, such as ZmHSF01 and ZmHSF06, whose mRNA increases sharply within a few minutes of thermal stimulation, while a few negatively regulated HSFS are also induced. ZmHSF20 is a core heat shock transcription factor discovered in recent years, and it belongs to the B2 class of HSF. Li et al. (2024) identified ZmHSF20 through heat stress transcriptome co-expression network analysis. Functional studies demonstrated that it plays a negative regulatory role in maize heat tolerance: overexpression of ZmHSF20 instead reduces the survival rate of seedlings, while knockout of ZmHSF20 significantly improves maize heat tolerance. Mechanism, ZmHSF20 directly binds to inhibit the promoter activity of another heat-resistant HSF - ZmHSF04, as well as the expression of cellulose synthetase ZmCesA2, thereby balancing the resource allocation between growth and defense (Figure 1). In addition to HSF, members of the bZIP transcription factor family are also involved in the thermal response. Research has found that the expression of the bZIP factor ZmGBF1 in corn increases at high temperatures and can bind to G-box elements to regulate downstream genes. Cao et al. (2021) reported that ZmGBF1 enhanced the heat resistance of maize by activating the key gene ZmCXE2 of the GA pathway. ZmNAC074 of the NAC family has been demonstrated to enhance heat tolerance in transgenic Arabidopsis thaliana, suggesting that its function in corn may be to promote the expression of heat-resistant related genes. In fact, co-expression analysis revealed that multiple transcription factor family nodes were enriched in the maize high-temperature response gene network: AP2/ERF, MYB, bHLH, NAC, HSF, etc. were all directly or indirectly involved in regulating the high-temperature stress response. For instance, ZmWRKY106 has been reported to simultaneously enhance the drought resistance and heat resistance of corn. ZmNF-YC13, as a nuclear factor Y subunit, overexpression driven by high-temperature induced promoters can activate multiple heat-resistant genes and increase the survival rate of plants. These findings suggest that different transcription factors work together to form a complex regulatory network for corn in response to heat stress. Among them, on the one hand, there are "emergency commanders" like HSF that directly induce protective genes such as HSP; on the other hand, there are relay nodes like bZIP and NAC that integrate ROS, hormones and other signals and regulate the expression of a wide range of downstream genes. It is worth noting that one of the research hotspots of Chinese scholars is precisely the transcriptional regulatory mechanism of corn under heat stress, which will also be a key entry point for future heat-tolerant genetic improvement.
.png) Figure 1 Heat tolerance is modulated by ZmHsf20 (Adopted from Li et al. 2024) Image caption: A) Construction of CRISPR/Cas9-mediated Zmhsf20 knockout lines. Two sgRNAs that specifically target ZmHsf20 were designed, leading to the identification of two mutants, Zmhsf20-1 and Zmhsf20-2. black rectangles exons, white rectangles Un-Translated Regions (UTRs) and horizontal black lines rectangles introns. B) Relative ZmHsf20 transcript levels in the leaves of V2 stage seedlings of ZmHsf20-OE and wild type (WT, KN5585). ACTIN was used as the internal control. The error bars are based on three independent experiments. The values are means ± Sd (n = 3 independent experiments). ***P < 0.001, one-way ANOVA. C) Representative photographs of V2 stage seedlings of WT, Zmhsf20-1, and Zmhsf20-2 grown at 28 °C/22 °C (top) or exposed to 45 °C HS treatment for 3 d followed by a 3-d recovery (bottom). Scale bar = 2 cm. D) Representative photographs of V2 stage seedlings of WT, ZmHsf20-OE #1, and ZmHsf20-OE #1 grown at 28 °C/22 °C (top) or exposed to 45 °C for 2 d followed by a 3-d recovery (bottom). Scale bar = 2 cm. E, F) Survival rate of seedlings after recovery at 28 °C/22 °C for 3 d in (C, D). The error bars are based on three independent experiments. The values are means ± Sd (n = 3 independent experiments). *P < 0.05, **P < 0.01, ***P < 0.001, one-way ANOVA. G, H) Ion leakage rate of V2 stage seedlings grown at 28 °C/22 °C exposed to 45 °C for 1d. The error bars are based on three independent experiments. The values are means ± Sd (n = 3 independent experiments). *P < 0.05, ***P < 0.001, one-way ANOVA. I, J) Representative photographs of leaves from V2 stage seedlings grown at 28 °C/22 °C, exposed to 45 °C for 1 d, and stained with DAB, scale bar = 1.5mm (Adopted from Li et al. 2024) |
3.3 Potential roles of non-coding RNAs in transcriptional regulation under heat stress
In addition to protein-coding genes, high-temperature stress can also cause significant changes in the expression profile of non-coding Rnas in corn, among which non-coding Rnas represented by micrornas (mirnas) play an important role in post-transcriptional regulation. miRNA is a type of 20-24 nt small RNA that mediates the cleavage or translation inhibition of target genes through base complementary pairing with target mrnas, thereby influencing gene expression levels. A large number of studies have shown that high temperatures can induce the up-regulation or down-regulation of miRNA expression in many plants and change the regulation of downstream stress-resistant genes. A variety of mirnas related to high-temperature responses have also been identified in corn. For instance, by using high-throughput sequencing, Qian et al. (2019) identified that approximately 40 mirnas, including miR156, miR159, miR160, and miR398, were significantly differentially expressed in the transcriptome under high-temperature stress at the seedling stage of maize. Among them, miR156 plays an important role in the high-temperature response of corn: its target is the SPL transcription factor family. Research has found that the specific upregulation of miR156 at high temperatures can inhibit the expression of the SPL gene, thereby enhancing the continuous expression of HSF and HSP and endowing plants with acquired heat-resistant "memory" ability. In contrast, the expression pattern of miR398 in corn differs from that in model plants: the expression changes of miR398 in corn under high-temperature stress are still uncertain, but in Arabidopsis thaliana, miR398 inhibits its target antioxidant enzyme genes such as CSD1 and CSD2, leading to the accumulation of ROS and the activation of the HF-HSP pathway, thereby enhancing heat tolerance. These speculations provide ideas for the function of corn miR398. Recent studies on corn hybrids have also shown that some mirnas may be involved in the regulation of pollen thermal sensitivity processes. For instance, miR159/319 indirectly affects male fertility by influencing auxin and GA pathways. In addition to miRNA, the role of long non-coding Rnas (lncrnas) in the high-temperature response of corn has also begun to attract attention. Du and Li (2024) summarized the functions of corn lncrnas in adverse conditions, pointing out that high temperatures can induce the expression of some lncrnas, which may act as molecular "sponges" to bind mirnas or recruit proteins, thereby regulating the expression of heat-resistant genes. For instance, it has been reported that certain lncrnas can form complexes with HSF, affecting its transcriptional activation efficiency for downstream HSP genes. Although the current understanding of the involvement of non-coding Rnas in the regulation of heat stress in maize is still relatively limited, there is already evidence suggesting that they are part of a complex gene regulatory network. In-depth exploration of the roles of miRNA and lncRNA in the heat tolerance of corn is not only expected to reveal new regulatory mechanisms, but also may provide novel heat resistance regulatory elements for molecular breeding. In the future, by constructing a non-coding RNA regulatory network at the whole-genome level of corn, it will help to comprehensively understand the transcriptional regulatory system of corn in response to high-temperature stress.
4 Heat Stress-Related Signaling Pathways and Molecular Networks
4.1 Initial responses of heat sensing and signal transduction
Plants' perception of high temperatures begins with a series of physicochemical changes at the cellular level, including enhanced membrane lipid fluidity, alterations in protein conformation, and metabolic imbalances. The cell membrane of corn seedlings is regarded as one of the heat receptors. When the temperature rises sharply, the lipid bilayer of the biofilm changes from the gel phase to the liquid crystal phase, and the membrane fluidity increases. This change is captured by membrane-binding receptors, triggering the opening of Ca2+ ion channels and a transient increase in cytoplasmic Ca2+. Calcium ions, as second messengers, play an amplifying and propagating role in the initial stage of thermal signal transmission. Studies have shown that within minutes of the onset of high-temperature stress in corn leaves, the concentration of free Ca2+ in the cytoplasm significantly increases, activating downstream signaling components such as calcium-dependent protein kinase (CDPK) and calmodulin (CaM). In addition to Ca2+, the unfolded protein response (UPR) is also an important mechanism for heat perception. When the temperature is too high, causing denaturation and aggregation of proteins, the molecular chaperone BIP on the endoplasmic reticulum detects an increase in misfolded proteins and then dissociates from the endoplasmic reticulum reticulum receptor IRE1, triggering the dimerization activation of IRE1. IRE1, as a signaling enzyme, further cleases the XBP1 transcript, triggering the UPR cascade reaction. There is evidence in corn that UPR is involved in heat stress responses: the ZmBIP gene is rapidly upregulated under high-temperature conditions, and some molecular chaperone genes downstream of the IRE1 pathway are also induced (Wang et al., 2024). Meanwhile, high temperatures can also alter the phase transition and fluidity of cell membranes, affecting the activity of receptor kinases on the membranes. For instance, thermal stimulation may enrich or depolymerize through membrane microdomains, activating mechanosensitive channel proteins or thermal shock receptor proteins on the cell membrane of corn, thereby mediating ion inflow and signal transduction. The primary perception of high temperature in corn involves multiple mechanisms such as membrane structure changes, Ca2+ signals, and UPR responses. These initial events rapidly convert changes in environmental temperature into biochemical signals within cells, laying the foundation for the activation of the heat stress response network. Rapid thermal signal perception and transduction ensure that corn seedlings can initiate protective response reactions immediately when high temperatures arrive, thereby reducing damage.
4.2 Regulatory roles of Ca²⁺, ABA, MAPK and other signaling pathways
During the process of heat stress signal transduction, the Ca2+ -mediated signaling pathway, the ABA-mediated hormone pathway, and the MAPK cascade are the three classic pathways for activating defense in plants such as corn. Firstly, Ca2+ plays a central role as a "second messenger". High-temperature induced cytoplasmic Ca2+ waves can be captured and activated by calcium-dependent protein kinases (CDPK). This type of CDPK can directly phosphorylate certain transcription factors or defense enzymes, thereby rapidly regulating gene expression. For instance, existing studies have shown that Ca2+-CaM signaling can activate ABA-induced antioxidant enzyme and nitric oxide (NO) synthesis, enhancing the antioxidant capacity and signal transduction efficiency of cells in the thermal response of corn. Secondly, the ABA pathway interacts and amplifies the thermal signal. After high temperature increases the ABA content, the downstream SnRK2 kinase cascade is activated through PYR/PYL receptors, thereby affecting the activity of a series of ABA response element (ABRE) binding proteins (such as ABI5, etc.). In corn, it has been reported that high temperatures can induce the joint participation of HSFA6b and ABI-like transcription factors: the MAPK pathway converges Ca2+ and ABA signals, phosphorylates transcription factors such as ABI and HSF, and enhances their transcriptional regulation of genes such as HSP and CyclinD, thereby improving the heat tolerance of corn. Thirdly, the MAPK cascading pathway serves as a convergence and amplification platform for various upstream signals. High temperature can activate MAPKKK through an unknown upstream kinase, and then successively activate MAPKK and MAPK through phosphoric acid. Some MAPK family members related to thermal response, such as ZmMPK3/6, were identified in corn, and their activity was enhanced under thermal stimulation (similar to the MPK3/6 of Arabidopsis thaliana involved in HSF regulation). The research by Gao et al. (2019) demonstrated that high-temperature stress can activate multiple transcription factors such as HSF and ABF, as well as the cell cycle regulatory factor CYCD5 through the MAPK cascade 1. Thereby promoting the heat tolerance of corn during the grain-filling period. MAPK can also regulate ROS signaling: for instance, in Arabidopsis thaliana, MPK6 phosphorylates the WRKY and ZAT transcription factors, the latter of which regulates the expression of antioxidant enzyme genes; A similar mechanism in corn remains to be further verified, but it is speculated that MAPK-ROS forms a positive feedback loop at high temperatures, helping to enhance stress resistance signals. Overall, the Ca2+ signal, ABA hormone and MAPK cascade constitute the core thoroughfares of the maize heat stress signal network. They are interlinked: Ca2+ and ABA signals can amplify each other through the MAPK pathway, and the effect of MAPK depends on the initial Ca2+ signal and ABA level. This multi-pathway coupled regulation ensures that corn can rapidly and harmoniously activate defense genes and adaptive physiological responses under heat stress, achieving efficient signal transmission from perception to response.
4.3 Gene co-expression network analysis revealing key regulatory modules
By leveraging high-throughput transcriptome data, researchers applied gene co-expression network analysis (methods such as WGCNA) to identify key gene modules and regulatory centers in the heat stress response of maize. By classifying genes with similar expression patterns under different high-temperature treatment conditions into one category of modules, some co-expressed gene groups closely related to heat-resistant phenotypes can be discovered. For instance, Cao et al. (2021) conducted a weighted co-expression network analysis of the transcriptomes of five maize inbred lines treated at 45 °C, dividing 17 062 differentially expressed genes into 19 modules. Among them, the "turquoise green" module contained 6 089 genes and was significantly upregulated under high-temperature stress, and was regarded as a key module for maize's heat tolerance response. The genes of this module are enriched in the pathways related to heat response and reactive oxygen species clearance, and contain multiple genes encoding heat shock proteins and antioxidant enzymes, suggesting that its function is directly related to heat tolerance. Network analysis further reveals that in the co-expression relationship of these genes, several transcription factor genes are in a "central" position. For instance, module enrichment analysis revealed that the transcription factor genes of the HSF, ERF and bZIP families were highly enriched in the corn high-temperature response module, belonging to the "hub" nodes in the network. This is consistent with the findings of Li et al. (2024): They conducted a heat stress time series transcriptome analysis on the maize inbred line B73 and identified a gene module highly correlated with heat response using WGCNA. In this module, HSF-like transcription factors were significantly enriched and had the highest connectivity. In this co-expression subnetwork with HSF as the hub, not only numerous HSP and defense genes are included, but also some signaling pathway elements and secondary metabolic genes are incorporated, reflecting the systemic response triggered by heat stress. In addition to the core regulatory module, the co-expression network can also help identify new candidate heat-resistant genes. Tang et al. (2023) compared the transcriptomes of heat-tolerant and sensitive maize inbred lines and conducted an association analysis of 142 heat-responsive core genes shared by both with known heat-tolerant QTLS. They found that 42 genes were located in the reported heat-tolerant QTL intervals, including multiple genes encoding transcriptional regulatory factors and molecular partners. These genes are very likely the key candidate genes that affect the heat tolerance of corn. In addition, network analysis can also reveal the regulatory relationships among genes. For instance, Li et al. (2024) proposed through network inference that ZmHSF20-ZmHSF4-ZmcesA2 constitutes a regulatory module: ZmHSF20 inhibits the expression of ZmHSF4 and ZmCesA2, while ZmHSF4 directly activates ZmCesA2, thereby jointly regulating the balance between cell wall synthesis and thermal defense. This example demonstrates that network analysis can concatenate scattered genes into meaningful regulatory units. The gene co-expression network provides an effective means for analyzing the complex regulatory relationship of high-temperature response in corn. By identifying the high-temperature upregulation module and the hub genes within it, researchers can identify the key regulatory modules and genes that contribute significantly to heat tolerance. On this basis, they can further carry out functional verification and breeding applications.
5 Expression Specificity and Dynamic Regulatory Features
5.1 Tissue-specific responses of roots, leaves, and stems under heat stress
There are significant differences in the sensitivity and response patterns of different tissues and organs of corn to high-temperature stress. During the seedling stage, the root system, leaves and stems respectively undertake functions such as water and nutrient absorption, photosynthesis and mechanical support. The impact of high temperature on them varies. Generally speaking, leaves, as organs directly exposed to solar heat radiation, are the most sensitive to high temperatures: high temperatures cause the stomata on leaves to close, transpiration to decrease, and the temperature to rise, making them more prone to scorching and a decline in photosynthetic rate. The root system, being in the soil, has a relatively stable temperature and a relatively high tolerance for short-term high temperatures. However, continuous high temperatures can also inhibit root growth and cause poor root hair development, thereby affecting water and nutrient absorption (Zhang and Xu, 2024). A single-cell transcriptome study by Wang et al. (2025) revealed that different cell types in corn roots respond to high temperatures to varying degrees. Among them, cortical cells are the most sensitive to high temperatures. High temperatures can significantly alter the gene expression profile of cortical cells and inhibit their division and elongation. This indicates that even within the root, the responses of each tissue layer to heat stress are specific. As a nutrient transport and support organ, the stem has a relatively insignificant direct response to high temperatures, but high temperatures may indirectly affect the stem by interfering with hormone transport and structural development. For instance, research has found that under the combined effect of high temperature and drought, the vascular bundle tissue and cell wall structure of corn stalks change, and their mechanical strength decreases. Co-expression network analysis also indicated that there were differences in the gene modules activated by different tissues under heat stress: the upregulated genes enriched in leaf tissues were mostly related to photosynthesis and heat shock proteins, while the upregulated genes enriched in root systems were more related to aquaporin and osmotic regulation (Huo et al., 2023). In addition, the impact of high temperatures on reproductive organs (such as inflorescences) is particularly crucial. Although this paper focuses on the seedling stage, it should be pointed out that the filaments and male spikes are extremely sensitive to high temperatures. High temperatures may lead to a decline in pollen vitality and a reduction in pollen release in male spikes, as well as fertilization obstacles in female spikes, thereby resulting in a significant reduction in yield. Under high-temperature stress, different tissues of corn each have their vulnerable links and main response mechanisms. Therefore, when studying the heat tolerance of corn, tissue specificity needs to be taken into consideration. For instance, in breeding, improvements can be made to the organs most vulnerable to heat damage (such as spikelets and leaves) to enhance the overall heat resistance of the plant. Meanwhile, in the design of research experiments, the physiological and molecular indicators of roots, stems and leaves should also be separately tested to comprehensively assess the heat resistance characteristics of the material.
5.2 Temporal expression dynamics of genes under different time points of stress
The response of corn to high temperatures is a dynamic process. Different durations of high-temperature stress will activate different gene expression programs. Usually, in the early stage of heat stress (within a few minutes to several hours), plants rapidly induce a batch of emergency response genes, such as the transcription of heat shock transcription factor and heat shock protein genes, which increases significantly within 15 to 30 minutes. This is a typical "instantaneous heat response". Subsequently, if the high-temperature stress persists, corn will enter a "sustained response" stage, and some metabolic pathway genes related to stress resistance (such as antioxidant enzymes, osmotic protective substance synthases, etc.) will maintain high levels of expression, while the HSP gene that initially rose sharply may gradually reach a stable state after several hours of high-temperature treatment. Li et al. (2024) conducted a time series transcriptome analysis on the maize B73 inbred line, comparing the expression at different time points such as 0.5 hours, 1 hour, 3 hours, and 6 hours of high-temperature treatment, and found that the set of genes upregulated at different times was different. Among them, the genes upregulated in the early stage (0.5 h-1 h) were mainly protective genes such as HSF and HSP; In the middle stage (about 3 hours), the upregulated genes expand to pathways such as antioxidation, hormone signaling, and glucose metabolism; In the late stage (6 hours and beyond), the expression of some genes related to cell repair and growth recovery is upregulated. This indicates that the heat tolerance response of corn is phased: first, it rapidly protects cells, then adjusts metabolic homeostasis, and finally attempts to resume growth. Another study also supports this dynamic rule: Jiang et al. (2020) observed through a time gradient experiment that the expression level of the HSP70 gene in corn seedlings soared within 15 minutes after the start of high temperature, reached a peak within 1 hour, and gradually decreased after 4 hours. However, the expression of the antioxidant enzyme gene was significantly upregulated 1-2 hours after high temperature and remained so for a relatively long time. In addition, pretreatment with high-temperature stress can induce a "thermal memory" effect, enabling some defense genes to remain highly expressed even after the stress is lifted, so as to respond more quickly when the temperature returns later. For example, although the expression levels of ZmHSP17 and ZmHSP101 genes decreased after the first high temperature, they were still higher than those of the untreated control, and could be upregulated more rapidly at the second high temperature (Li et al., 2022). Other studies have shown that different heat-resistant varieties have differences in the dynamics of gene expression: heat-resistant materials tend to activate defense genes earlier and maintain their high levels of expression for a long time, while the response of sensitive materials may be lagging and not long-lasting. In conclusion, the expression of maize heat response genes has distinct temporal dynamic characteristics. From instantaneous response to continuous response and then to adaptation and recovery, the gene networks at each stage are interconnected. This suggests that when studying the heat tolerance mechanism of corn, we should combine the time dimension and capture the molecular events at key time points. In breeding selection, indicators such as the residual level of gene expression after thermal shock treatment can also be used to evaluate the thermal memory ability and thermal durability of materials.
5.3 Coordinated mechanisms of spatial and temporal gene regulation
The resistance of corn to high-temperature stress is the result of the coordinated action of its various tissues at different time scales, that is, spatial regulation and temporal regulation are closely combined. The division of labor and complementarity of different parts of the plant in high temperatures enable corn to enhance its overall heat resistance. For instance, when high temperatures strike, leaves rapidly synthesize HSP and other substances to protect their own cellular functions, while the root system can send drought/heat combined signals to the above-ground parts through hormones such as ABA, causing stomata to close and reducing water loss. This synergy between roots and leaves helps the plant survive short-term extreme high temperatures. On a longer time scale, the heat responses at different periods also need to be coordinated: the protective and restorative mechanisms strengthened during the seedling stage may lay the foundation for heat resistance in the later reproductive stage. For instance, the heat shock proteins and other substances produced by high-temperature pretreatment during the seedling stage remain at a certain level within the plant. When the plant enters the tasseling and flowering stage, these chaperone proteins may still exist, thereby enhancing the pollen's tolerance to high temperatures. This reflects the influence of temporal "memory" on spatial resistance. In addition, the specificity of gene expression in space is itself regulated by temporal factors. Take the male ears of corn as an example. The spike differentiation period and the flowering period are the two time Windows that are most sensitive to heat. The damage caused by high temperature to the male ears during these two stages is greater than that during the vegetative growth period. Studies show that high temperatures during the spikicle differentiation period can reduce the number of small flowers in male spikes, while high temperatures during the flowering period can lower pollen vitality and the amount of pollen released. Therefore, only by protecting the right tissue at the right time can the best heat resistance effect be achieved. This requires precise spatio-temporal expression regulation (Liu et al., 2023). For instance, the continuous expression of the ZmHSFA2 gene in corn leaves is crucial for heat tolerance, but in organs such as filaments and grains, it may only need to be expressed at specific stages. For instance, some mirnas and hormone signals may move over long distances between roots, stems and leaves, playing a coordinating role across tissues. Cross-tissue signals and local gene regulation jointly form the regulatory network of heat resistance throughout maize plants. In future research, integrating multi-omics data from different tissues and at different time points is expected to construct a spatio-temporal regulation model for corn's response to high-temperature stress, revealing the contribution of each tissue to heat resistance at each growth stage. This also has guiding significance for formulating agricultural defense strategies: for instance, cultivation measures can be taken to avoid high temperatures during the most sensitive flowering and grain stage of corn, and during the seedling stage, plants can be trained to acquire heat tolerance memory. Furthermore, the synergy of spatio-temporal expression also reminds us that in the evaluation of heat-tolerant breeding, we should comprehensively examine the heat resistance performance of materials at different growth stages and in different organs to screen out truly comprehensively heat-tolerant strains. The heat tolerance mechanism of corn is a complex spatio-temporal coordinated regulation process, which requires not only the mutual cooperation of various organs in space but also continuous defense at each stage in time, jointly ensuring the survival and growth of plants in high-temperature adverse conditions (Chen et al., 2024).
6 Candidate Gene Identification and Application Prospects
6.1 Identification and functional annotation of significantly differentially expressed genes under heat stress
With the aid of transcriptomics technology, a large number of maize genes (DEGs) that were significantly differentially expressed under high-temperature stress were identified. These DEGs constitute the main gene pool of maize's heat response and also the starting point for exploring heat-tolerant functional genes. In the transcriptome analysis of corn seedlings or flowering tissues, it is often possible to detect that thousands of genes are upregulated or downregulated due to high-temperature treatment. For instance, Qian et al. (2019) reported that under short-term high temperature (42 ℃ treatment for 6 hours) during the seedling stage of corn, approximately 2 000 genes were significantly upregulated and about 1 500 genes were significantly downregulated, involving multiple functional categories such as heat shock proteins, antioxidant enzymes, osmotic regulatory substances, and signal proteins. Through bioinformatics annotation, these DEGs can be classified into several key pathways. Some upregulated DEGs belong to the "protein folding and repair" pathway (rich in HSP genes), some belong to the "reactive oxygen species scavenging" pathway (rich in SOD, APX and other genes), and some are involved in "hormone signaling" and "secondary metabolism", etc. For instance, the 142 core heat-stress response genes identified by Tang et al. (2023) were enriched in GO categories such as protein folding, ROS metabolism, and carbohydrate metabolism, indicating that these processes are significant in the heat tolerance of maize. Functional annotation and enrichment analysis of differentially expressed genes not only help identify known heat-resistant pathways (such as HSP, ABA, Ca2+ signaling, etc.), but may also discover new pathways that have not received attention before. For instance, some studies have found that the gene expression of the aquaporin family in corn also changes at high temperatures, suggesting that water transport may also be regulated under heat stress (He et al., 2022). For instance, some secondary metabolism-related genes (such as flavonoid synthase) are upregulated at high temperatures and may play a role in membrane stabilization or antioxidation. By conducting association analysis between DEGs and known heat-resistant QTLS, the range of candidate genes can be further narrowed down. Tang et al. (2023) compared the transcriptomes of heat-tolerant and sensitive maize, overlapping the common DEGs of both with heat-tolerant QTLS in maize yield and flowering period, and identified 42 differential genes located in the QTL region, such as genes encoding heat shock proteins, transcription factors, and cytoprotective enzymes. These genes are very likely the factors that affect the heat tolerance of corn. High-temperature induced DEGs identification provides a rich gene resource bank for the study of the heat tolerance mechanism of maize. Among these genes, there are many key genes that have been verified by existing research (such as ZmHSF, ZmHSP, ZmCAT1, etc.), as well as many new genes with unknown functions. Further functional annotation and cluster analysis of these candidate genes can serve as a basis for selecting important candidate genes. For instance, focus on screening genes that are specifically upregulated in heat-resistant varieties but do not increase in sensitive varieties, as well as genes that remain highly expressed at all stages of heat treatment. Such genes are more likely to be directly related to heat-resistant phenotypes. Next, functional experiments (such as genetic complementarity, gene editing, etc.) need to be conducted to verify its biological effects and select the genes that truly possess heat-resistant functions from them.
6.2 Promoter characteristics and regulatory patterns of heat-tolerant candidate genes
For the candidate genes for corn heat tolerance obtained through screening, it is necessary to conduct in-depth research on their expression regulatory mechanisms, among which the cis-acting elements of the promoter and the upstream regulatory factors are the key entry points. The promoter regions of many heat-tolerant related genes are rich in typical thermal response elements (such as HSE, ABRE, DRE, etc.), revealing their transcriptional regulatory patterns. For instance, most HSP gene promoters have repetitive sequences of the thermal shock element HSE. The HSF protein can bind to HSE at high temperatures, thereby activating the transcription of the HSP gene. Experiments have demonstrated that the absence of HSE in the ZmHSP17.4 promoter significantly reduces its heat-induced expression level (Yao et al., 2019). For instance, the promoters of some heat-resistant antioxidant enzyme genes (such as ZmCAT1) contain ABA-responsive elements ABRE and DRE/CRT elements, indicating that they may be jointly regulated by ABA signals and DREB-like transcription factors. Research has found that under high-temperature conditions, ABA accumulation can activate genes such as ZmCAT1 by binding to ABRE through ABI transcription factors, while genes like DREB2A, lacking their own activation domains, need to collaborate with HSF to effectively promote the transcription of heat-resistant genes. Another example is the promoter of ZmHSF20 itself. Li et al. (2024) reported that there are HSF binding sites in its promoter region, suggesting that ZmHSF20 may be controlled by a higher-order HSF regulatory network and participate in negative feedback regulation. For key candidate genes, it is also necessary to clarify their upstream signaling pathways. Taking the ZmNAC transcription factor gene as an example, if its promoter carries the abolic acid response element ABRE, it is speculated that when the ABA signal rises, the expression of the NAC gene is enhanced, and thus the NAC protein further regulates the downstream HSP or antioxidant genes, achieving an ABA-mediated heat resistance pathway. Similarly, genes containing Ca2+/CaM response elements (such as the CGCG cassette) can be regulated by calcium signaling pathways. Studies have demonstrated that the CaM binding element on the ZmHSP70 promoter has a positive effect on its thermally induced expression (Song et al., 2016). In addition to the cis-type element of the promoter, heat-resistant genes often involve complex transcriptional regulatory networks. For instance, the aforementioned ZmHSF20 module: ZmHSF20 is heat-induced and, together with other HSFS, forms a regulatory network that can feedback inhibit the expression of ZmHsf4 and certain defense genes. Therefore, when analyzing the regulatory patterns of key genes, co-expression networks and genetic analysis should be combined to clarify which transcription factors directly act on the promoters of candidate genes and which downstream targets the candidate genes regulate. The interaction between candidate gene promoters and upstream transcription factors can be verified through experiments such as yeast single-hybrid (Y1H) and dual-luciferase reporting. For instance, Cao et al. (2021) utilized DAP-seq technology to discover that ZmGBF1 directly binds to the ZmCXE2 promoter and matches the G-box sequence, thereby clarifying the pattern by which ZmGBF1 regulates genes in the GA pathway. This type of research is crucial for the application of heat-resistant genes: by understanding their promoter characteristics and regulatory factors, it is possible to achieve a more ideal expression pattern of the target gene at high temperatures through promoter engineering or targeted editing. For instance, placing key HSP genes under the control of thermal shock promoters in corn (such as the ZmHSP17.9 promoter), or knocking out binding sites that negatively regulate their expression, may enhance the heat tolerance performance of plants.
6.3 Case study: Transcriptome-based comparative study of heat-tolerant maize varieties
Specific cases can further illustrate the application value of transcriptomics in the study of corn heat tolerance and variety improvement. The study selected a pair of corn hybrids with significant differences in heat tolerance and compared their transcriptomes under high-temperature stress. After high-temperature treatment during the flowering period, heat-tolerant varieties (such as PF5411-1) and sensitive varieties (such as LH150) showed yield differences: the number of grains per panicle and the weight of a thousand grains in the former were less affected, while the latter significantly reduced yield. Transcriptome analysis identified a set of heat-upregulated genes shared by the two varieties, as well as their respective specific expression changes. It was found that the common response genes of the two varieties were concentrated in the basic defense pathways, such as heat shock proteins and antioxidation-related genes, which were all upregulated, indicating that these are common heat response pathways. However, in heat-tolerant varieties, some genes that were not significantly changed in sensitive varieties were specifically upregulated, such as the gene encoding Trehalose-6-phosphate synthase, which might enhance the carbohydrate protective effect of heat-tolerant varieties. It was also found that the expression levels of several transcription factors (such as HSF and DREB types) in heat-resistant varieties were higher than those in sensitive varieties. These differential genes are believed to endow heat-resistant varieties with stronger heat regulation capabilities. On the other hand, some genes that may be related to growth inhibition and premature aging were induced in sensitive varieties. For instance, the expression of the abscisic acid synthase gene was higher than that in heat-tolerant varieties, indicating that high temperatures caused sensitive varieties to overly enter the "survival mode", sacrificing yield formation. Based on these findings, researchers further utilized co-expression network analysis to identify several key genes that might determine the differences in heat tolerance among varieties. For instance, the regulatory modules formed by specifically upregulated ZmHsfA2 and ZmDREB2 in heat-tolerant varieties are believed to enhance the expression of downstream defense genes, thereby reducing the harm of high temperature to pollen and grain development (Figure 2) (Chen et al., 2023). On the contrary, the overactivated ABA/ ethylene signaling module in sensitive varieties leads to accelerated senescence, affecting the yield. Finally, the study functionally verified the identified candidate genes: by knocking out an unknown functional gene that was specifically highly expressed in heat-tolerant varieties with CRISPR/Cas9, the heat tolerance of the transgenic plants significantly decreased, demonstrating that this gene has a positive effect on the heat resistance of corn. This case demonstrates that transcriptome comparison can effectively reveal the differences in molecular responses between heat-resistant and sensitive varieties, from which potential key heat-resistant factors can be identified. When applying these genes to breeding, molecular marker-assisted selection of materials carrying favorable alleles can be adopted, or the genotypes of sensitive varieties can be directly improved through transgenic/gene editing. For instance, the favorable allele of ZmHsfA2 discovered above can be tracked and selected in the breeding population through molecular markers, thereby enhancing the heat tolerance of the new combination. Introducing the heat-resistant version of the ZmDREB2 gene into sensitive varieties may also enhance their heat resistance. It can be seen that the variety comparison study combining transcriptomics and functional genomics methods has provided clear molecular targets and improvement ideas for the cultivation of heat-tolerant corn, accelerating the process of genetic improvement.
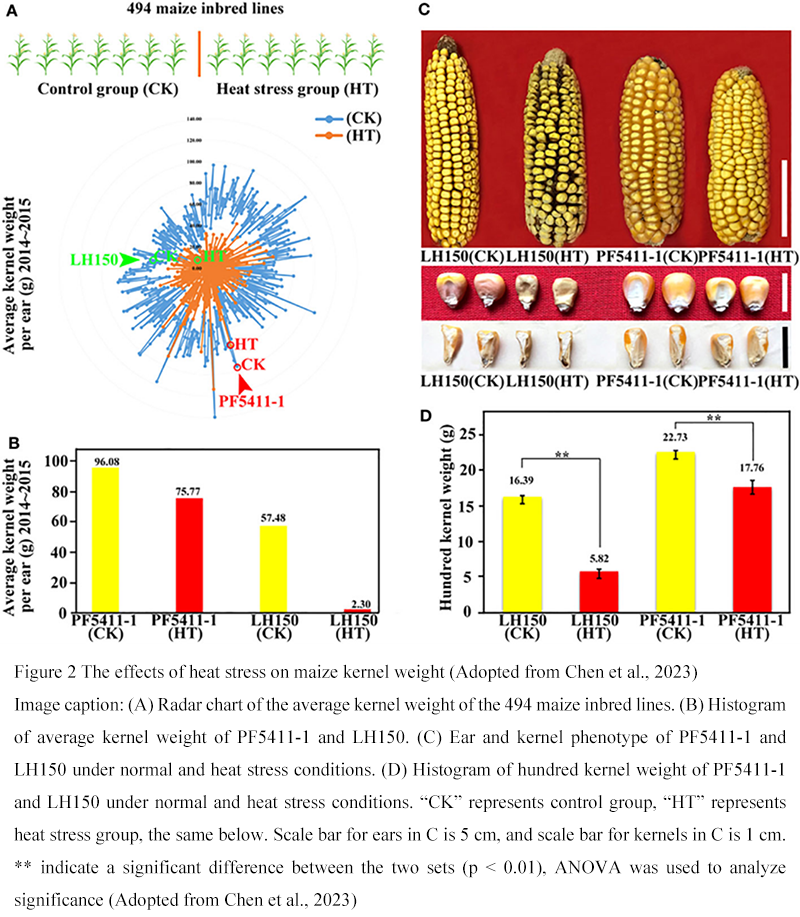 Figure 2 The effects of heat stress on maize kernel weight (Adopted from Chen et al., 2023) Image caption: (A) Radar chart of the average kernel weight of the 494 maize inbred lines. (B) Histogram of average kernel weight of PF5411-1 and LH150. (C) Ear and kernel phenotype of PF5411-1 and LH150 under normal and heat stress conditions. (D) Histogram of hundred kernel weight of PF5411-1 and LH150 under normal and heat stress conditions. “CK” represents control group, “HT” represents heat stress group, the same below. Scale bar for ears in C is 5 cm, and scale bar for kernels in C is 1 cm. ** indicate a significant difference between the two sets (p < 0.01), ANOVA was used to analyze significance (Adopted from Chen et al., 2023) |
7 Conclusions and Future Perspectives
Recent studies have initially constructed a molecular regulatory map of corn's response to high-temperature stress. This study summarizes the multi-level mechanisms of high-temperature response in corn seedlings: From a physiological perspective, high temperatures lead to a decline in photosynthetic efficiency and an increase in respiratory loss in corn, disrupt the balance of reactive oxygen species and antioxidant systems within the body, and disrupt hormone levels, thereby inhibiting the normal growth and development of the plants. At the molecular level, corn alleviates heat damage by rapidly upregulating heat shock protein genes and activating genes related to antioxidant enzymes and protective substances. Meanwhile, multiple heat-response transcription factors (such as HSF, bZIP, NAC, etc.) synergistically regulate the transcription of downstream defense genes, forming a complex gene regulatory network. These transcriptional regulatory activities rely on the effective conduction of high-temperature signals within cells, including pathways such as Ca2+ transients, ABA and ROS signal mediation, and MAPK cascade amplification. Analysis of the gene co-expression network reveals that factors such as HSF are at the hub position of the maize heat response network, and many stress-resistant genes jointly constitute modular regulatory units. The gene expressions in different tissues and at different time stages are specific yet interrelated, ensuring the coordinated global and local heat resistance responses of plants. This study sorted out a large number of candidate heat-resistant genes identified by transcriptome technology and analyzed the action modes of some key genes (such as ZmHSF20, ZmGBF1, etc.) in combination with functional experiments. These achievements mark an important progress in the research on the high-temperature response mechanism of corn: a molecular map containing each link of "thermal perception - signal transduction - transcriptional regulation - physiological response" is gradually becoming clear. This map provides a framework for us to understand the genetic basis of corn heat tolerance and helps to subdivide heat tolerance traits into locatable and detectable molecular indicators (such as the expression level of specific genes, the content of specific metabolites, etc.). Overall, corn has demonstrated typical thermal response patterns, similar to model plants yet with its own characteristics: for instance, the HSF and HSP systems of corn function similarly to those of Arabidopsis thaliana, but as a C4 crop, corn has unique features in carbon metabolism regulation and organ-level responses (such as the issue of heat tolerance in the panicle). This knowledge laid the foundation for the next step of in-depth research.
Although significant progress has been made in the research on the heat tolerance mechanism of corn, many problems remain unsolved and some technical challenges are also faced in the research. Firstly, in terms of genetic identification, although a large number of heat-tolerant related candidate genes have been discovered through transcriptome and association analysis, the functions of many of these genes remain unclear. For a crop like corn, which has a large genome and high functional redundancy, functional verification is rather difficult. At present, the number of key genes with verified functions is limited, and most candidate genes (especially non-coding Rnas and metabolic regulatory factors) still lack in-depth research. This leads to an incomplete understanding of the regulatory network of corn heat tolerance. Secondly, in terms of the regulatory mechanism, the signal crosstalk and coordination mechanism during the multi-level response process of corn remain unclear. For instance, how do signaling pathways such as Ca2+, ABA, and MAPK integrate with each other? Is there a cascade regulation or feedback loop among different transcription factors? These issues have only been reported sporadically so far, and systematic analysis still needs to be advanced. In addition, how to coordinate and unify the responses among different tissues and at different developmental stages is also a major challenge. Corn is a cross-pollinating crop, and its reproductive growth is extremely sensitive to heat. However, there are currently few studies on the correlation between heat tolerance during the vegetative growth period and the reproductive period, and there is still a lack of theoretical guidance on how to achieve heat tolerance improvement throughout the entire growth period. Secondly, in terms of breeding application, there is still a distance to go before the genes discovered in the laboratory can be applied to field varieties. Heat tolerance is a complex quantitative trait. The effect of a single gene is often limited, and it requires the aggregation of multiple favorable alleles to produce a significant improvement effect. However, multi-gene aggregation is prone to sacrificing other agronomic traits. How to improve heat tolerance while maintaining yield is a difficult point in breeding practice. In addition, high-temperature stress often occurs simultaneously with other adverse conditions such as drought. The heat and drought tolerance mechanisms of corn overlap and conflict (for example, closing stomata is beneficial for heat tolerance but reduces photosynthetic yield). How to balance the responses to multiple stresses is also a problem that needs to be considered. Technically, accurately simulating field heat stress conditions for research remains challenging. High temperatures in the field are often accompanied by changes in the day-night cycle, light conditions and moisture levels. The constant temperature treatment under laboratory control conditions differs from the actual environment. This may lead to some experimental results being difficult to reproduce in the field. Therefore, future research needs to develop stress test systems that are closer to real environments, such as intelligent field greenhouses and mobile greenhouses, to enhance the application relevance of research results. Finally, the corn genome is large and polyploid, with redundant functional genes and a complex genetic background, which increases the difficulty of gene mining. The application of gene editing in corn also faces problems such as low transformation efficiency and needs further improvement. In conclusion, the research on the heat tolerance of corn is still in the stage of deepening from macroscopic phenomena to microscopic mechanisms. We not only need to discover "which genes" are involved, but also clarify "how these genes work together". Solving these problems will help to comprehensively reveal the heat tolerance mechanism of corn and transform it into stable and high-yield genetic improvement results.
Facing the complex trait of heat tolerance in corn, the future research trend will be the integration of multi-level omics data and systems biology analysis. Single transcriptome studies have revealed a wealth of information, but if genomic, proteomic, metabolomic and other data can be combined, it is expected to more comprehensively depict the response network of corn to high-temperature stress. For instance, through genomics and association analysis, key genetic variation sites that affect heat tolerance can be located, while proteomics can identify functional proteins that undergo modification or abundance changes at high temperatures, and metabolomics reflects the alterations in the physiological metabolic state of corn under heat stress. Combining these omics data with transcriptomics can establish a system regulatory model of gene-protein-metabolites. For instance, for a key gene that is upregulated at high temperatures, proteomic data can be used to confirm whether the encoded protein has accumulated or undergone post-translational modifications, and metabolomic data can be used to verify whether its downstream metabolites have changed accordingly, thereby verifying the heat tolerance function of the gene from multiple aspects. In recent years, some studies have begun to explore this direction. The research group led by Li Lin from Huazhong Agricultural University and others have constructed the first multi-omics integration network for corn, uniformly analyzing three-dimensional genomic, transcriptomic, proteomic and other information. They found that approximately 30 000 corn genes jointly participate in stress response through different levels of association. It can be foreseen that the introduction of omics integration in heat resistance research will help discover regulatory relationships that cannot be detected by a single omics. For instance, some genes that do not show differential expression in the transcriptome may play a role in heat stress through improved translation efficiency or enhanced protein stability, which requires proteomic data to capture. Or, changes in the metabolome can suggest adjustments in biochemical pathways not covered by the transcriptome. For instance, the content of certain osmotic protective substances increases under heat stress, and the key enzyme genes responsible for their synthesis may not be detected as DEG. However, by discovering this phenomenon through the metabolome and then delve deeper into the transcriptome, regulatory clues can be found. In the future, new technologies such as single-cell sequencing and spatial transcriptomics can be utilized to analyze the expression changes of different cell types in corn under heat stress, and combined with traditional omics to more precisely construct a heat tolerance regulatory network. At the application level, multi-omics integration can also assist in breeding decisions. For instance, combined genome-transcriptome analysis can be used for the association between genotypes and expression types, and to screen out genes that have both excellent alleles and are highly expressed under heat conditions for breeding markers. Proteomic-metabolome binding can be used to identify reliable heat tolerance biomarkers for rapid field evaluation of variety heat tolerance. In conclusion, with the development of sequencing and analysis technologies, cross-omics integration will enable us to understand the complex system network of corn heat tolerance at an overall level, and thereby guide more rational and efficient genetic improvement strategies. Through the integration of different disciplines, the biological research on corn stress resistance is moving from a "one-dimensional" to a "multi-dimensional" approach. In the future, it is expected to achieve precise design and modification of corn heat tolerance, contributing to ensuring global food production and responding to climate change.
Acknowledgments
We appreciate Dr. Huang from the Hainan Institution of Biotechnology for her assistance in references collection and discussion for this work completion.
Conflict of Interest Disclosure
The authors affirm that this research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Cao L.R., Wang G.R., Zhang X., Wei L.M., Wei X., Zhang Q.J., Deng Y.Z., Wang Z.H., and Lu X.M., 2021, Genome-wide identification and analysis of HSP90 gene family in maize, Crops, 5: 28-34.
https://doi.org/10.16035/j.issn.1001-7283.2021.05.005
Chen Q., Ying Q.H., Lei K.Z., Zhang J.M., and Liu H.Z., 2024, The integration of genetic markers in maize breeding programs, Bioscience Methods, 15(5): 226-236.
https://doi.org/10.5376/bm.2024.15.0023
Chen Y., Du T., Zhang J., Chen S., Fu J., Li H., and Yang Q., 2023, Genes and pathways correlated with heat stress responses and heat tolerance in maize kernels, Frontiers in Plant Science, 14: 1228213.
https://doi.org/10.3389/fpls.2023.1228213
Doğru A., 2021, Effects of heat stress on photosystem II activity and antioxidant enzymes in two maize cultivars, Planta, 253(85): 1-15.
https://doi.org/10.1007/s00425-021-03611-6
Du Q.G., and Li W.X., 2024, Research progress in the regulation of development and stress responses by long non-coding RNAs in maize, Chinese Bulletin of Botany, 59(6): 950-962.
https://doi.org/10.11983/CBB24075
Gao J.Y., Wang S.F., Zhou Z.J., Wang S.W., Dong C.P., Mu C., Song Y.X., Ma P.P., Li C.C., Wang Z., He K.W., Han C.Y., Chen J.F., Yu H.D., and Wu J.Y., 2019, Linkage mapping and genome-wide association reveal candidate genes conferring thermotolerance of seed-set in maize, Journal of Experimental Botany, 70(18): 4849-4864.
https://doi.org/10.1093/jxb/erz171
He R., Su H., Wang X., Ren Z., Zhang K., Feng T., Zhang M., Li Z., Li L., Zhuang J., Gong Z., Zhou Y., and Duan L., 2022, Coronatine promotes maize water uptake by directly binding to the aquaporin ZmPIP2;5 and enhancing its activity, Journal of Integrative Plant Biology, 65(3): 703-720.
https://doi.org/10.1111/jipb.13432
Huo Z.G., Zhang H.Y., Li C.H., Kong R., and Jiang M.Y., 2023, Review on high temperature heat damage of maize in China, Journal of Applied Meteorological Science, 34(1): 1-14.
https://doi.org/10.11898/1001-7313.20230101
Jiang L., Hu W., Qian Y., Ren Q., and Zhang J., 2020, Genome-wide identification, classification and expression analysis of the Hsf and Hsp70 gene families in maize, Gene, 745: 145348.
https://doi.org/10.1016/j.gene.2020.145348
Kim K.H., and Lee B.M., 2023, Effects of climate change and drought tolerance on maize growth, Plants, 12: 3548.
https://doi.org/10.3390/plants12203548
Li Y.F, Huang Y.M., Sun H.Y., Wang T.Y., Ru W., Pan L.L., Zhao X.M., Dong Z.B., Huang W., Jin W.W., 2022, Heat shock protein 101 contributes to the thermotolerance of male meiosis in maize, Plant Cell, 34(10): 3702-3717.
http://dx.doi.org/10.1093/PLCELL/KOAC184
Li Z., Li Z.R., Ji Y.L., Wang C.Y., Wang S.F., Shi Y.T., Le J., and Zhang M., 2024, The heat shock factor 20-HSF4-cellulose synthase A2 module regulates heat stress tolerance in maize, Plant Cell, 36(7): 2652-2667.
http://dx.doi.org/10.1093/plcell/koae106
Liu M., Zhou Y., Sun J., Mao F., Yao Q., Li B., Wang Y., Gao Y., Dong X., Liao S., Wang P., and Huang S., 2023, From the floret to the canopy: high temperature tolerance during flowering, Plant Communications, 4: 100629.
https://doi.org/10.1016/j.xplc.2023.100629
Pandey S., Divakar S., and Singh A., 2024, Genome editing prospects for heat stress tolerance in cereal crops, Plant Physiology and Biochemistry, 215: 108989.
https://doi.org/10.1016/j.plaphy.2024.108989
Qian Y.X., Ren Q.Y., Zhang J., and Chen L., 2019, Transcriptomic analysis of the maize (Zea mays L.) inbred line B73 response to heat stress at the seedling stage, Gene, 692: 68-78.
https://doi.org/10.1016/j.gene.2018.12.062
Razzaq A., Mustafa G., Ali M., Khan M.S., and Joyia F.A., 2021, CRISPR-mediated genome editing in maize for improved abiotic stress tolerance, Molecular Breeding in Wheat, Maize and Sorghum, 23: 405-420.
https://doi.org/10.1079/9781789245431.0023
Song J., Weng Q., Ma H., Yuan J., Wang L., and Liu Y.H., 2016, Cloning and expression analysis of the Hsp70 gene ZmERD2 in Zea mays, Biotechnology & Biotechnological Equipment, 30(1): 219-226.
https://doi.org/10.1080/13102818.2015.1131625
Tang B., Geng C.J., Zeng Q., Guo H.L., Li H., Cao Z.Y., Deng L.C., Peng M., Zhou H., and Chen Z.H., 2023, Kernel transcriptome analysis of maize inbred lines in response to high temperature stress, Acta Agriculturae Boreali-Sinica, 38(4): 11-19.
https://doi.org/10.7668/hbnxb.20193984
Wang T., Feng J.L., and Zhang C., 2024, Research Progress on molecular mechanisms of heat stress affecting the growth and development of maize, Chinese Bulletin of Botany, 59(6): 963-977.
https://doi.org/10.11983/CBB24049
Wang T., Wang F., Deng S., Wang K., Feng D., Xu F., Guo W., Yu J., Wu Y., Wuriyanghan H., Li S.T., Gu X., Le L., and Pu L., 2025, Single-cell transcriptomes reveal spatiotemporal heat stress response in maize roots, Nature Communications, 16: 177.
https://doi.org/10.1038/s41467-024-55485-3
Yao Q.L., Chen F.B., Li W.B., and Fang P., 2019, Screening for physiological indexes of maize inbred lines under heat stress, Journal of Maize Sciences, 8: 84-88.
https://doi.org/10.13597/j.cnki.maize.science.20190614
Zhang X., and Xu M.L., 2024, Adaptation of maize to various climatic conditions: genetic underpinnings, Bioscience Evidence, 14(3): 122-130.
https://doi.org/10.5376/be.2024.14.0014
.png)
. PDF(771KB)
. FPDF(win)
. FPDF(mac)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Jiamin Wang
. Xian Zhang
. Guifen Wang
Related articles
. Corn
. High-temperature stress
. Transcriptome
. Thermal response gene
. Heat resistance
Tools
. Email to a friend
. Post a comment


