Linkage Mapping of Quantitative Trait Loci for Traits Promoting Aerobic Adaptation on Chromosome 8 in Indica Rice (Oryza sativa L.) 
 Author
Author  Correspondence author
Correspondence author
Rice Genomics and Genetics, 2015, Vol. 6, No. 6 doi: 10.5376/rgg.2015.06.0006
Received: 29 May, 2015 Accepted: 03 Aug., 2015 Published: 20 Aug., 2015
Kharb A., Sandhu N., Jain S., and Jain R. K., 2015, Linkage Mapping of Quantitative Trait Loci for Traits Promoting Aerobic Adaptation on Chromosome 8 in indica Rice (Oryza sativa L.), Rice Genomics and Genetics, Vol.6, No.6 1-5 (doi: 10.5376/rgg.2015.06.0006)
Identification of major effect QTLs for traits promoting aerobic adaptation using molecular markers can greatly enhance the efficacy of breeding programs to develop high yielding, direct seeded, water efficient rice varieties. The Filial (F2) population derived from HKR47 high-yielding low-land indica rice variety, and MAS26, aerobic adapted rice variety, was developed and analyzed for the identification of larger and consistent effect QTLs for yield, yield attributing and root traits under aerobic cultivation conditions. The population displayed large variation for all the physio-morphological traits including grain yield per plant and root traits. Phenotypic correlation analysis revealed that grain yield per plant showed positive correlation with root length (r2=0.279), fresh root weight (r2=0.232) and dry root weight (r=0.269). A total of 803 SSR markers, distributed on 12 rice chromosomes, were analyzed for parental polymorphism survey; of these 125 (about 16%) displayed polymorphism. NTSYS-pc UPGMA tree cluster analysis and two-dimensional PCA scaling showed scattering of the F2 population between the two distinct parental genotypes; the population was inclined towards MAS26. Composite interval mapping (CIM) analysis revealed a total of six QTLs (qTN8.1, qTN8.2, qTN8.3, qTGW8.1, qYPP8.1 and qRL8.1) on chromosome 8 (within a region of 24.9 cM) which individually explained 13.7%~27.3% of the phenotypic variation. Chromosome 8 also possesses QTL for aroma and kernel elongation demonstrating that it might be difficult to introgress these QTL promoting aerobic adaptation in Basmati rice.
Water scarcity has critical impact on world’s food self-sufficiency and security. Almost one-fifth of the world's population, live in areas of physical scarcity of water and almost one quarter of the world's population, face economic water shortage (where countries lack the necessary infrastructure to take water from rivers and aquifers). Water use has been growing at more than twice the rate of population increase in the last century (FAO, 2007, http://www.fao.org/nr/water/docs/escarcity.pdf) . According to the International Water Management Institute (IWMI), agriculture, which accounts for about 70% of global water withdrawals, is constantly competing with domestic, industrial and environmental uses for a scarce water supply (Sentilinger, 2013, http://thewaterproject.org/ water-scarcity-and-agriculture) . Indian agriculture totally depends on monsoon and the agriculture sector in India uses 85% of the country’s available water. Less than 3% of the world’s water is fresh and even of this 3% over 2.5% frozen, locked up as glaciers and only 0.5% fresh water is available to man for various purposes. In such a scenario, if we continue to apply current water management practices, by 2050, the global agricultural sector will need to double the amount of water to feed the world. According to the UN Convention to Combat Desertification (UNCCD), with the existing climate change scenario, almost half the world's population will be living in areas of high water scarcity by 2030 (UN, 2014, http://www.un.org/waterforlifedecade/scarcity.shtml ).
Rice, world’s most important food crop that feeds over half of the global population, is the biggest consumer of fresh water diverted for irrigation. Globally rice is grown over an area of about 164.7 million ha with an annual production of 745.7 million tonnes (FAO, 2013, http://faostat3.fao.org/browse/Q/QC/E ). More than 90% of the world’s rice is grown and consumed in Asia where 60% of the earth’s population lives. China and India, which account for more than one-third of global population, produce over half of the world's rice. In India, area under rice cultivation is 43.5 million ha with an annual production of 159.2 million tonnes (FAO, 2013, http://faostat3.fao.org/browse/Q/QC/E). India is the largest user of groundwater in the world - if the trend of indiscriminate exploitation continues, over a quarter of the global total and about 60 per cent of aquifers in India will be in a critical condition in another 15~20 years. We need a shift from “land productivity” without concern for water use to “water productivity,” that is, getting the highest yield out of every drop of water used in agriculture. Some important questions arise here are: Should we continue with our present rice cultivation practices? Do we need to have a most productive and effective water saving cultivation method? Is it easy to breed for aerobic adapted rice varieties? In addition to the problem of water scarcity an equally important aspect of sustainable rice cultivation is the availability of labor to grow rice under traditional cultivation conditions as the rural population moves away from farming to work in urban areas in industry.
Improved root system is very important for maintaining crop yields, when plants are grown in insufficient supplies of water or nutrients. Plants having deeper root system colonize a large soil volume and improve the water uptake from the lower layers where water is expected to be available; this helps to maintain a good plant water potential which lead to zero or small yield decline under water limited conditions (Mumbani and Lal, 1983). Aerobic rice refers to a cultivation system in which rice is dry direct seeded in well-tilled leveled fields with uniform slope under unpuddled conditions. Aerobic rice have long and thick root system (Yadav et al., 1997; Ling et al., 2002), erect leaves, medium height, better water use efficiency and maintain high biomass and harvest index under upland conditions. In addition to reducing water use during land preparation and limiting seepage, percolation, and evaporation, aerobic rice had about 51% lower total water use and 32%~88% higher water productivity, expressed as gram of grain per kilogram of water, than flooded rice (Bouman et al., 2005), less labor use (Wang et al., 2002) and reduction of greenhouse gas emission from rice field (Mandal et al., 2010).
Even if several QTL for root traits (Zhang et al., 2001; Liu et al. 2008; Courtois et al. 2009) and grain yield under drought stress (Bernier et al., 2007; Venuprasad et al., 2009) have been identified, however, identification of consistent effect QTL for traits promoting adaptation to aerobic cultivation may be helpful in introducing varieties with high yield potential.
In our earlier study, 35 QTLs associated with 14 traits were mapped on chromosomes 1, 2, 5, 6, 8, 9, and 11 in MASARB25 × Pusa Basmati 1460 and 14 QTL associated with 9 traits were mapped on chromosomes 1, 2, 8, 9, 10, 11, and 12 in HKR47 × MAS26. Two QTL (qGY8.1 and qGY2.1) and one QTL (qGY2.2) were identified for grain yield under aerobic conditions in the mapping populations MASARB25 × Pusa Basmati 1460 and HKR47 × MAS26, respectively (Sandhu et al., 2013).
In this paper, we report the net house evaluation of a F2 population derived from the cross between “HKR47” (high yielding) and “MAS26” (aerobic) varieties of indica rice for yield, yield attribute traits and root traits. F2 population has been used for linkage mapping of QTLs linked with traits promoting aerobic adaptation using already-mapped SSR markers.
1 Results
1.1 Phenotypic variation for agronomic traits
Aerobic rice cultivar, MAS26, out yielded the lowland variety, HKR47, under dry direct-seeded aerobic cultivation conditions. Root length, fresh & dry root weight and root thickness were higher in MAS26 compared to HKR47. A large variation for plant height (52.3~100.5 cm), effective number of tillers/plant (1~13), panicle length (17.7~27.9 cm), grain length/breadth ratio (2.41~3.31), grain weight (13.6~25.8 g) and yield per plant (1~26.2 g) were observed in HKR47 × MAS26 F2 population. Root traits also showed wide range of variation; root length, fresh and dry root weight and root thickness ranged from 18.1~72.4 cm, 3.21~57.8 g, 1.17~17.1g and 1.77~5.5 cm, respectively (Table 1; Figure 1). Compared to the aerobic rice parental variety MAS26 (RL-51.7 cm, DRW -7.48 g), 88 plants had greater root length (51.8~72.4 cm) and 70 plants had greater dry root weight (7.53~17.1 g).
.jpg) Table 1 Mean and range for various agronomic and root traits in HKR47 x MAS26 F2 population under net house conditions |
1.2 Genetic analysis
Out of 803 SSR markers widely distributed on 12 rice chromosomes, a total of 125 (about 16%) polymorphic SSR markers were used for the F2 population genotyping. A DNA fingerprint database of selected 94 HKR47 × MAS26 F2 plants was prepared using 125 SSR markers. On an average, 50.2 % alleles were from MAS26 and 49.8 % alleles were from HKR47 in all 94 F2 plants. To demonstrate the genetic relationship among the F2 plants and parental rice varieties, allelic diversity data was used for cluster tree analysis, NTSYS-pc. The parental rice varieties HKR47 and MAS26 had low similarity coefficient and bifurcate at coefficient value of 0.49. All the 94 F2 plants did not cluster into major groups but showed more similarity to MAS26. Two dimensional PCA scaling exhibited that two parental genotypes were quiet distinct whereas 94 F2 plants interspersed between the two parental lines with distribution of some plants towards MAS26 (Table 2; Figure 2; Figure 3).
 Figure 2 Dendrogram (NTSYS-PC) displaying diversity among 94 HKR47 × MAS26 F2 plants and parental genotypes using allelic diversity data at 125 SSR loci |
 Figure 3 Two dimensional PCA scaling displaying diversity among 94 F2 plants (HKR47 × MAS26) and parental genotypes using allelic diversity data at 125 SSR loci |
A total of six QTL associated with four traits (Table 3; Figure 4; Figure 5) were detected. Three QTL for effective number of tillers per plant (qTN8.1, qTN8.2 and qTN8.3 at map positions 36.0, 55.2, 60.9 cM, respectively), one for thousand grain weight (qTGW8.1;59.1 cM), one for grain yield per plant (qYPP8.1;60.1 cM) and one for root length (qRL8.1, 43.7 cM) were identified. All the six QTL were mapped on chromosome 8 within a region of 24.9 cM (36~60.9 cM) (Figure 4; Figure 5). QTL qYPP8.1 with RM72 being the peak marker with an R2 value of 20.2% and qRL8.1 with RM8243 being the peak marker with an R2 value of 13.7% were identified for grain yield and root length under aerobic cultivated conditions, respectively. qYPP8.1 had maximum LOD score of 5.06. RM8020 and RM72 showed significant association with effective number of tillers/plant and RM8243 with root length. The QTL qTN8.1, qTN8.2, qTN8.3, qTGW8.1, qYPP8.1 and qRL8.1 accounted for 16.1, 21.0, 17.3, 27.4, 20.2 and 13.7 % phenotypic variation, respectively. Of the six QTL identified, one QTL (qRL8.1) with an additive effect of 3.9 was from MAS26, the remaining five QTL were from HKR47.
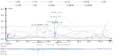 Figure 4 QTL likelihood curves of LOD score of all the detected QTLs on chromosome 8 in HKR47 × MAS26 F2 population |
 Figure 5 Chromosomal locations of quantitative trait loci (QTL) identified via microsatellite marker analysis in HKR47 × MAS26 F2 population |
2 Discussion
Groundwater is the primary source for irrigation, but overexploitation has resulted in a rapid decline of groundwater levels (Zhang et al., 2003). To tackle this situation, it has become essential to develop alternate ways of rice cultivation that require less water with no reduction in grain yield and quality. Several strategies are being pursued, including the development of water-efficient aerobic rice system, to reduce water requirement for rice cultivation including aerobic rice (Bouman, 2002).
Root system is considered as one of the important physiological parameter to struggle against the water scarce conditions. Under low-moisture stress, root characters are considered to be a vital component to fight against mechanism since they contribute to the regulation of plant growth and extraction of water and nutrients from deeper layers (Fukai and Cooper, 1995). Among the root morphological traits, maximum root length, dry root weight and root thickness were found to be associated with drought resistance in upland conditions. In the present study, root length, root biomass and root thickness were found to be higher in aerobic rice variety (MAS26) than in drought susceptible parent (HKR47). MAS26 had 47% higher root length, 37% higher dry root weight and 16% higher root thickness than HKR47. Eighty-eight and seventy plants had better root length and dry root weight than MAS26, respectively. It has been reported that a cultivar having more root number, high fresh and dry root can explore more soil volume for effective absorption of water (Amudha et al., 2009). Results also revealed that deep roots are associated with water stress tolerance in aerobic rice. Several research groups have reported identification of markers linked to the genes/QTL for root traits such as maximum root length, basal root thickness, dry root weight, total root weight and their potential role in water use efficiency or drought tolerance (Price et al., 1997; Yadav et al. 1997; Li et al. 2005; Toorchi et al., 2007; Liu et al. 2008; Qu et al., 2008).
Kato et al. (2010) studied root response to aerobic conditions in rice and observed that total root length under aerobic and near-saturated conditions was 10%~30% of that under flooding. Matsuo et al., (2009) reported that root number, fresh and dry root were higher in Sensho (upland variety) compared to Koshihikar (lowland variety) in hydroponic culture. Martin et al., (2007) reported that the rice varieties (ADT 39 and PMK 3) that were suitable for cultivation under aerobic conditions had longer and deeper root system compared to the other rice varieties. Increased root length allows roots to penetrate hard pans characteristic of some lowlands, thickness and density improves water uptake by producing more and larger root branches (Ingram et al., 1994). The improvement of upland rice through a deeper root system is thought by many to be a promising way to increase water uptake, and ultimately grain yield, under water stress conditions (Fukai and Cooper, 1995).
Although yield potential of aerobic rice genotypes are lower than the low-land indica rice varieties being cultivated under conventional flooded conditions but the cultivation of aerobic rice is quite promising where water is too scarce to grow lowland rice. In aerobic rice, the combined amount of rainfall and irrigation water from sowing to harvest varied from 470 to 650 mm, compared with 1200-1300 mm in lowland rice (Martin et al., 2007). In the present study, we reported that aerobic rice variety (MAS26) and several segregating HKR47 × MAS26 F2 plants performed well in terms of grain yield per plant as compared to HKR47 under aerobic cultivated conditions. Yield per plant of HKR47 × MAS26 F2 population ranged between 1~26.22 g (HKR47 – 7.42 g, MAS26 – 8.34 g). Several studies supported our results, aerobic rice varieties yielded higher than lowland varieties under aerobic conditions (Bouman 2002; Xiaoguang et al., 2005; Kato et al., 2009; Murthy et al., 2011; Babu et al., 2011).
In order to evaluate the association between root and yield traits phenotypic correlation coefficient was calculated (Table 2). In the present study, yield per plant showed a significant positive correlation with plant height, effective number of tillers per plant, panicle length and thousand grain weight. Earlier studies also reported significant association between yield and plant height, panicle length and number of tillers (Girish et al., 2006; Nagaraju et al., 2013). It is necessary to study root morphological character’s influence on the grain yield and yield morphological traits. Under water limited conditions root system tries to develop adaptability that either may be beneficiary for the plants or may be tradeoff for yield. We identified a significant and positive correlation between yield per plant and root traits; root length (0.279, p=0.01), fresh root weight (0.232, p=0.01) and dry root weight (0.269, p=0.01). This shows that a well developed root system will help the plant in maintaining high plant water status which may leads to increase in yield potential under aerobic conditions. Plant height showed positive correlation with dry shoot weight (0.305, p=0.01). Positive association between plant height and shoot dry weight, maximum root length, root thickness and root dry weight has been observed by Ekanayake et al. (1985); and Kanbar and Shashidhar (2004). The interrelationships between root morphological characters and yield-related traits clearly showed the role of root traits in rice plant adaptability under aerobic conditions.
In the present study, we identified three QTL for effective number of tillers per plant, one for thousand grain weight, one for grain yield/plant and one for root length. A notable aspect of this study was that all the six QTL were mapped on chromosome 8 in a region of 24.9 cM segment (36-60.9 cM). QTL qRL8.1 with a peak at RM8243 with an R2 value of 13.7% associated with root length was identified. In this region three QTL for root length were reported by Sandhu et al., (2013) in the mapping populations MASARB25 × Pusa Basmati 1460 and HKR47 × MAS26. QTL qGY8.1 for grain yield was reported to be located near to the qRL8.1 (Sandhu et al., 2013). Sandhu et al. (2014) reported QTL for root hair length (qRHL8.1 at 43.3 cM) in the Aus276/3*IR64 BC2F4 population. Qu et al. (2008) identified two QTL for root number, one for fresh root weight and three for root volume on chromosome 8 using a total of 120 RILs derived from cross between japonica upland rice IRAT109 and paddy rice Yuefu. Li et al. (2005) reported QTL for maximum root length, dry root weight and ratio of dry root weight to dry shoot weight using double haploid population from a cross between two japonica cultivars under three different conditions (upland, lowland and upland in pvc pipes). Courtois et al., (2003) identified a QTL for maximum root length on chromosome 8 using RILs derived from cross between IAC165 and Co39 rice varieties. Priyadarshini et al., (2013) evaluated the effectiveness of the root QTL on grain yield and water use efficiency in a set of 116 near isogenic lines (NILs) derived from cross IR64 and Azucena populations which were screened for grain yield and water use efficiency under severe reproductive-stage drought stress. These Collocating chromosomal loci governing different root and grain yield traits may provide breeders a better prospect to introgress such regions together as a unit in order to develop high yielding aerobic adapted cultivars.
QTL qYPP8.1, for grain yield per plant at 60.1 cM with RM72 being the peak marker with an R2 value of 20.2% was reported in this study. Sandhu et al. (2013) reported QTL for grain yield at 72.2 cM in the mapping population MASARB25 × Pusa Basmati 1460. Sandhu et al., (2014) reported QTL for grain yield adjacent to qYPP8.1 in the Aus276/3* IR64 BC2F4 population. Adjacent to qYPP8.1, Vikram et al. (2012) also reported QTL for grain yield under drought, qDTY8.1, in Basmati334/ Swarna. Hanamaratti et al. (2007) reported QTL on chromosome 8 associated with relative yield and drought susceptibility index in IR64 × Binam-derived NILs under drought stress. Bernier et al., (2008) reported a minor QTL for grain yield at 28 cM position. Zhang et al., (2006) mapped a QTL related to drought tolerance on chromosome 8. Sandhu et al. (2013) reported QTL for effective number of tillers per plant, adjacent to qTN8.2 and qTN8.3, in the mapping population MASARB25 × Pusa Basmati 1460 and two QTL for effective number of tillers per plant with an R2 value of 26.4% and 29.3%, respectively, in the mapping population HKR47 × MAS26. The QTLs identified earlier were not identified in the present study. It can be hypothesized that there was a strong G × E interaction among traits. It may be possible because of the small size of the population. The co-location of QTLs inherited from different parents as shown by DPE (Table 3) suggest that it will be necessary to fine map the particular region, which assist the precise introgression of the QTLs in a marker assisted selection (MAS) program.
QTL analysis is an unbiased investigation of the genes affecting a particular trait which provides information on the location of important loci for the traits under study without any prior knowledge on the genes involved and reveals their possible genetic effects leading to phenotypes of interest. In the present study, six QTL (for four traits; yield per plant, effective number of tillers per plant, grain weight and root length) were identified within a region of 24.9 cM between RM8020 and RM72 markers on chromosome 8. A significant positive correlation has also been observed between yield per plant and effective number of tillers per plant, grain weight and root length. It might be possible that this 24.9 cM region is contributing towards root, yield and yield attributing traits and promoting adaptation to aerobic cultivated conditions. This region may facilitate marker assisted breeding to develop aerobic adapted high yielding rice varieties. It is quite interesting that this study led to the identification of a number of QTL on chromosome 8 which also possesses QTL for aroma and kernel elongation in Basmati rice. These two QTLs (aroma and cooked kernel elongation) are linked and present in the vicinity of QTL identified in the present study within 69~78.6 cM region on chromosome 8 (Jain et al., 2004; Jain et al., 2006). It indicates that it would be difficult to introgress these QTL promoting aerobic adaptation in Basmati rice.
3 Materials and Methods
3.1 Plant material
Seed harvested from the F1 plants obtained from the cross between “HKR47” (used as female) and “MAS26” (used as male) varieties of indica rice, were used to raise F2 population in pots during 2012 kharif season in the net house of Department of Molecular Biology, Biotechnology and Bioinformatics at CCS Haryana Agricultural University, Hisar. While “HKR47” is a low-land high yielding indica rice variety unadapted to cultivation in aerobic conditions whereas “MAS26” is an aerobic rice variety developed at University of Agricultural Sciences, Bangalore.
3.2 Net house evaluation and trait measurements
HKR47 × MAS26 F2 population comprising of 184 plants were sown in the pots (one plant per pot) in a net house and raised to maturity under aerobic cultivated conditions. Seeds were sown in pots of 12ʺ height. Vermicompost manure was added in the soil at the time of pot filling. The pots were irrigated with one liter of water for the first fifteen days, then at an interval of three days up to panicle emergence and then at an interval of two days after panicle emergence. Yoshida nutrient solution was given to the plants growing in pots (500 mL per pot) after an interval of 21 and 70 days from the sowing date. The pots were kept weed-free by manual weeding. At physiological maturity, data was recorded on agronomic traits, plant height in cm (PH), effective number of tillers per plant (TN), panicle length in cm (PL), 1,000-grain weight in g (TGW), length/breadth ratio (L/B), and grain yield in g (YPP). Plant height was measured from the stem base to the tip of highest panicle (excluding awn). Fully developed tillers bearing panicles of each plant were counted at the time of maturity. Length of panicle was measured from the base of panicle until the tip of the last grain. The length and breadth of three seeds from each plant was recorded using digital Vernier Caliper. For grain weight determinations, 100-grain (dehusked) samples were taken from the harvested grains to compute 1000-grain weight. Grain yield per plot was recorded after harvesting, threshing, and drying to moisture content adjusted to 14%. The data on root length (RL, cm), fresh and dry root weight (FRW and DRW in g), root thickness (RT in cm), and dry shoot weight (DSW in g) was recorded and analyzed. For the measurement of root traits, plants were removed from the pots and gently washed. Root length was measured using a centimeter scale. The roots and shoots were then separated by cutting from the stem base. For fresh root weight, the roots were blotted gently with a blotting paper to remove any free surface moisture and then weighed immediately. For dry weight, the roots and shoots were dried in an oven set to low heat (50°C) overnight, and then cooled in a dry environment. Once cooled, weighed on a scale. The thickness of the root crown was measured using a vernier caliper.
3.3 Statistical analysis
The data was subsequently analyzed using OPSTAT (http://hau.ernet.in/opstat.html) to determine the variability and phenotypic (r) correlation coefficient analysis. Phenotypic correlation coefficients were tested against standardized tabulated significant value of r with (n–2) degree of freedom as per the procedure (Fisher and Yates, 1963).
3.4 Molecular characterization and QTL mapping
A total of 94 F2 plants selected on the basis of root traits, grain weight and grain yield (15% best and 15% worst) covering the entire range of these phenotypic traits were used for identification and mapping of QTLs associated with traits promoting aerobic adaptation. DNA was isolated from the leaf tissues of F2 plants using CTAB DNA isolation (Saghai-Maroof et al., 1984). Molecular marker analysis was carried out using 125 polymorphic SSR primers by ethidium bromide stained polyacrylamide gel electrophoresis (PAGE). PCR products from SSR analysis were scored visually for presence or absence of bands; data was scored as 1 (present) and 0 (absent) for each of the SSR locus. Genetic similarities between the genotypes were measured by the similarity coefficient based on the proportion of shared electromorphs using ‘Simqual’ sub-program of NTSYS-PC (Version 2.02 Exeter Software, Setauket, NY, USA) package (Rohlf, 1993). The resultant distance matrix data was used for two-dimensional scaling of rice genotypes using two-dimensional Principal Component Analysis (PCA). QTL analysis was done by using Win QTL cartographer version 2.5 (Shengchu Wang, Christopher J. Basten and Zhao-Bang Zeng, 2012, http://statgen. ncsu.edu/qtlcart/WQTLCart.htm) via composite interval mapping (CIM). The threshold log likelihood ratio (LOD) score was estimated empirically with 300 times permutations at a significant level of P=0.05.
Author’s contributions
AK was involved in the conception of the experiment, analysis, interpretation of the data, and drafting the article and final approval of the version to be published; NS was involved in revising manuscript content critically and final approval of the version to be published; SJ was involved with the analysis, interpretation of the data, in the critical revision of the manuscript and final approval of the version to be published; RKJ was involved in the design of the experiment, the critical revision of the manuscript and final approval of the version to be published.
Acknowledgements
The authors thank the Department of Science and Technology, New Delhi, India (DST No. SR/SO/PS/0041/2009), for providing financial support for this study.
References
Amudha K., Thiyagarajan K., Robin S., Prince S.J.K., Poornima R., and Suji K.K., 2009, Heterosis under aerobic condition in rice, Electron. J. Plant Breed., 1(4): 769-775
Babu N.N., Hittalmani S., Shivakumar N., and Nandini C., 2011, Effect of drought on yield potential and drought susceptibility index of promising aerobic rice (Oryza sativa L.) genotypes, Electron. J. Plant Breed., 2(3): 295-302
Bernier J., Kumar A., Venuprasad R., Spaner D., and Atlin, G.N., 2007, A large-effect QTL for grain yield under reproductive-stage drought stress in upland rice, Crop Sci., 47: 507-516
http://dx.doi.org/10.2135/cropsci2006.07.0495
Bernier J., Kumar A., Venuprasad R., Spaner D., Verulkar S., Mandal N.P., Sinha P.K., Peeraju P., Dongre P.R., Mahto R.N., and Atlin G., 2008, Characterization of the effect of a QTL for drought resistance in rice, qtl12.1, over a range of environments in the Philippines and eastern India, Euphytica, 166(2): 207-217
http://dx.doi.org/10.1007/s10681-008-9826-y
Bouman B.A.M., 2002, Aerobic Rice (Han Dao): a new way of growing rice in water short areas, Mol. Gen. Genet., 4: 53-61
Bouman B.A.M., Peng S., Castaneda A.R., and Visperas R.M., 2005, Yield and water use of irrigated aerobic rice systems, Agric. Water Manage., 74: 87-105
http://dx.doi.org/10.1016/j.agwat.2004.11.007
Courtois B., Ahmadi N., Khowaja F., Price A.H., Rami J.F., Frouin J., Hamelin C., and Ruiz M., 2009, Rice Root Genetic Architecture: Meta-analysis from a Drought QTL Database, Rice, 2: 115-128
http://dx.doi.org/10.1007/s12284-009-9028-9
Courtois B., Shen L., Petalcorin W., Carandang S., Mauleon R., and Li Z., 2003, Locating QTL controlling constitutive root traits in the rice population IAC 165 × Co39, Euphytica, 134: 335-345
http://dx.doi.org/10.1023/B:EUPH.0000004987.88718.d6
Ekanayake I.J., O’toole J.C., Garrity D.P., and Masajo T.M., 1985, Inheritance of root characters and their relations to drought resistance in rice, Crop Sci., 25: 927-933
http://dx.doi.org/10.2135/cropsci1985.0011183X002500060007x
Fisher R.A., and Yates F., eds., 1963, Statistical tables for biological, agricultural and medicinal research, 6th Ed., Oliver and Boyd, Edinburgh, London, pp.63
Fukai S., and Cooper M., 1995, Development of drought-resistant cultivars using physio-morphological traits in rice, Field Crop Res., 40: 67-86
http://dx.doi.org/10.1016/0378-4290(94)00096-U
Girish T.N., Gireesha T.M., Vaishali M.G., Hanamareddy B.G., and Hittalmani S., 2006, Response of a new IR50/Moroberekan recombinant inbred population of rice (Oryza sativa L.) from an indica x japonica cross for growth and yield traits under aerobic conditions, Euphytica, 152: 149-161
http://dx.doi.org/10.1007/s10681-006-9190-8
Hanamaratti N.G., 2007, Identification of QTL for physiological and productivity traits under drought stress and stability analysis in upland rice (Oryza sativa L.), Dissertation for Ph.D., University of Agricultural Sciences, Supervisor: Salimath P.M., pp.59-62
Ingram K.T., Bueno F.D., Namuco O.S., Yabao E.B., and Beyrouty C.A., 1994, Rice root traits for drought resistance and their genetic variation, In: Kirk G.J.D. (ed), Rice Roots: Nutrient and Water Use, International Rice Research Institute, Manila, Philippines, pp.67-77hanamaratti
Jain N., Jain S., Saini N., and Jain R.K., 2006, SSR analysis of chromosome 8 regions associated with aroma and cooked kernel elongation in Basmati rice, Euphytica, 152: 259–273
http://dx.doi.org/10.1007/s10681-006-9212-6
Jain S., Jain R.K., and McCouch S.R., 2004, Genetic analysis of Indian aromatic and quality rice (Oryza sativa L.) germplasm using panels of fluorescently-labeled microsatellite markers, Theor. Appl. Genet., 109: 965-977
http://dx.doi.org/10.1007/s00122-004-1700-2
Kanbar A., and Shashidhar H.E., 2004, Correlation and path analysis for root morphological traits in indica x indica population of rice (Oryza sativa L.), Crop Res., 27(1): 94-98
Kato Y., Nemoto K., and Yamagishi J., 2009, QTL analysis of panicle morphology response to irrigation regime in aerobic rice culture, Field Crop Res., 114: 295-303
http://dx.doi.org/10.1016/j.fcr.2009.08.014
Kato Y., Okami M., Tajima R., Fujita D., and Kobayashi N., 2010, Root response to aerobic conditions in rice, estimated by Comair root length scanner and scanner-based image analysis, Field Crop Res., 118(2): 194-198
http://dx.doi.org/10.1016/j.fcr.2010.04.013
Li Z.C., Mu P., Li C.P., Zhang H.L., Li Z.K., Gao Y.M., and Wang X.Q., 2005, QTL mapping of root traits in a doubled haploid population from a cross between upland and low-land japonica rice in three environments, Theor. Appl. Genet., 110: 1244-1252
http://dx.doi.org/10.1007/s00122-005-1958-z
Ling Z.M., Li Z.C., Yu R., and Mu P., 2002, Agronomic root characters of upland rice and paddy rice (Oryza sativa L.), J. Chin. Agri. Univ., 7: 7-11
Liu L., Mu P., Li X., Qu Y., Wang Y., and Li Z., 2008, Localization of QTL for basal root thickness in japonica rice and effect of marker-assisted selection for a major QTL, Euphytica, 164: 729-737
http://dx.doi.org/10.1007/s10681-008-9695-4
Mandal, D.K., Mandal C., Raja P., and Goswami S.N., 2010, Identification of suitable areas for aerobic rice cultivation in the humid tropics of eastern India, Curr. Sci., 99: 227-231
Martin G., James Padmanathan P.K., and Subramanian E., 2007, Identification on suitable rice variety adaptability to aerobic irrigation, J. Agric. Biol. Sci., 2: 2
Matsuo N., Ozawa K., and Mochizuki T., 2009, Genotypic differences in root hydraulic conductance of rice (Oryza sativa L.) in response to water regimes, Plant Soil, 316: 25-34
http://dx.doi.org/10.1007/s11104-008-9755-5
Mumbani B., and Lal R., 1983, Response of upland rice varieties to drought stress, Plant Soil, 73(1): 73-94
http://dx.doi.org/10.1007/BF02197758
Murthy K., Kumar A., and Hittalmani S., 2011, Response of rice (Oryza sativa L.) genotypes under aerobic conditions, Electron. J. Plant Breed., 2(2): 194-199
Nagaraju C., Sekhar M.R., Reddy K.H., and Sudhakar P., 2013, Correlation between traits and path analysis coefficient for grain yield and other components in rice (Oryza sativa L.) genotypes, J. Appl. Biol. Pharm. Technol., 4(3): 137-142
http://dx.doi.org/10.1007/s001220050541
Price A.H., Virk D.S., and Tomas A.D., 1997, Genetic dissection of root growth in rice (Oryza sativa L.) I: a hydroponic screen, Theor. Appl. Genet., 95: 132-142
Priyadarshini S.K., Shivanna H., and Hittalmani S., 2013, Response of NIILs with single and multiple QTL for root length on grain yield and water use efficiency in rice (Oryza sativa L) under aerobic condition, Int. J. Agr. Environ. Biotechnol., 6(3): 403-411
Qu Y., Mu P., Zhang H., Chen C.Y., Gao Y., Tian Y., Wen F., and Li Z., 2008, Mapping QTL of root morphological traits at different growth stages in rice, Genetica, 133: 187-200
http://dx.doi.org/10.1007/s10709-007-9199-5
Rohlf J., ed., 1993, Numerical taxonomy and multivariate analysis system NTSYS-pc, 18th Ed., Exeter Software, Stony Brook, New York, pp.18-35
Saghai-Maroof M.A., Soliman K.M., Jorgensen R.A., and Allard R.W., 1984, Ribosomal spacer length polymorphism in barley: Mendelian inheritance, chromosomal location and population dynamics, Proc. Natl. Acad. Sci., 81: 8014-8019
http://dx.doi.org/10.1073/pnas.81.24.8014
Sandhu N., Jain S., Kumar A., Mehla B.S., and Jain R., 2013, Genetic variation, linkage mapping of QTL and correlation studies for yield, root, and agronomic traits for aerobic adaptation, BMC Genet., 14: 104-119
http://dx.doi.org/10.1186/1471-2156-14-104
Sandhu N., Torres R.N., Cruz M.T.S., Maturan P.C., Jain R., Kumar A., and Henry A., 2014, Traits and QTL for development of dry direct-seeded rainfed rice varieties, J. Exp. Bot., 66(1): 225-244
http://dx.doi.org/10.1093/jxb/eru413
Toorchi M., Shashidhar H.E., and Hittalmani S., 2007, Tagging QTL for maximum root length in rainfed lowland rice by combined selective genotyping and STMs markers, J. Food Agric. Environ., 5(2): 209-210
Venuprasad R., Bool M.E., Dalid C.O., Bernier J., Kumar A., and Atlin G.N., 2009, Genetic loci responding to two cycles of divergent selection for grain yield under drought stress in a rice breeding population, Euphytica, 167: 261–269
http://dx.doi.org/10.1007/s10681-009-9898-3
Vikram P., Mallikarjuna Swami B.P., Dixit S., Helaluddin A., Sta Cruz M.T., Singh A.K., Guoyou Y., and Kumar A., 2012, Bulk segregant analysis: an effective approach for mapping consistent-effect drought grain yield QTL in rice, Field Crop Res., 134: 185-192
http://dx.doi.org/10.1016/j.fcr.2012.05.012
Wang H., Bouman B.A.M., Zhao D., Wang C., and Moya P.F., 2002, Aerobic rice in northern China: opportunities and challenges, In: Bouman B.A.M., Hengsdijk H., Hardy B., Bindraban P.S., Tuong T.P., and Ladha J.K. (eds.), Water-wise rice production, International Rice Research Institute, Los Baños, Philippines, pp.143-154
Xiaoguang Y., Bouman B.A.M., Huaqi W., Zhimin W., Junfang Z., and Bin C., 2005, Performance of temperate aerobic rice under different regimes in North China, Agric. Water Manage., 74(2): 107-122
http://dx.doi.org/10.1016/j.agwat.2004.11.008
Yadav R., Courtois B., Huang N., and McLaren G., 1997, Mapping genes controlling root morphology and root distribution in a doubled-haploid population of rice, Theor. Appl. Genet., 94: 619-632
http://dx.doi.org/10.1007/s001220050459
Zhang J., Zheng H.G., Aarti A., Pantuwan G., Nguyen T.T., Tripathy J.N., Sarial A.K., Robin S., Babu R.C., Nguyen B.D., Sarkarung S., Blum A., and Nguyen N.T., 2001, Locating genomic regions associated with components of drought resistance in rice: comparative mapping within and across species, Theor. Appl. Genet., 103: 19-29
http://dx.doi.org/10.1007/s001220000534
Zhang X., Zhou S., Fu Y., Su Z., Wang X., and Sun C., 2006, Identification of a drought tolerant introgression line derived from Dongxiang common wild rice (O. rufipogon Griff.), Plant Mol. Biol., 62: 247-259
http://dx.doi.org/10.1007/s11103-006-9018-x
Zhang X.Y., Dong P., and Hu C.S., 2003, Conserving groundwater for irrigation in the North China Plain, Irrigation Sci., 21: 159-166
. PDF(459KB)
. FPDF
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. A. Kharb
. N. Sandhu
. S. Jain
. R.K. Jain
Related articles
. Aerobic rice
. Linkage mapping
. Microsatellite marker
. QTL
. Root
. Yield
Tools
. Email to a friend
. Post a comment

.jpg)
.jpg)


