 Author
Author  Correspondence author
Correspondence author
Triticeae Genomics and Genetics, 2024, Vol. 15, No. 1 doi: 10.5376/tgg.2024.15.0006
Received: 08 Jan., 2024 Accepted: 12 Feb., 2024 Published: 21 Feb., 2024
Xu Q., and Cai R.X., 2024, Impact of domestication on nucleotide diversity in barley, Triticeae Genomics and Genetics, 15(1): 56-65 (doi: 10.5376/tgg.2024.15.0006)
As a widely cultivated crop worldwide, barley (Hordeum vulgare) has a long history of domestication and has had a profound impact on agricultural production and human life. With the development of molecular biology technology, research on nucleotide diversity in barley has gradually deepened, providing important basis for understanding the genetic structure and domestication mechanism of barley. This study aims to analyze the impact of domestication on nucleotide diversity in barley. By comparing and analyzing the nucleotide diversity of wild barley and cultivated barley, it is found that population bottlenecks and targeted breeding during domestication have a profound impact on genetic structure. Meanwhile, the changes in adaptive genes and genomic structure of barley caused by domestication were explored, revealing the impact of domestication mechanisms on crop genetics. This study aims to enhance the understanding of the genetic mechanism of barley domestication, and provide scientific basis for variety improvement and genetic resource protection. In addition, it has reference value for other crop domestication and genetic diversity research, and is of great significance for promoting the development of agricultural biotechnology.
Domestication is the process by which wild species are adapted to human needs through selective breeding. This process has been pivotal in the development of agriculture, transforming wild plants and animals into forms that are more useful to humans. Domestication has led to significant changes in the genetic makeup of species, resulting in traits that enhance yield, ease of harvest, and suitability for cultivation. These changes are often referred to as the "domestication syndrome" which includes traits such as reduced seed dispersal, increased seed size, and changes in plant architecture (Smýkal et al., 2018).
Barley (Hordeum vulgare) is one of the oldest and most important cereal crops, with evidence of its cultivation dating back over 10 000 years in the Fertile Crescent (Kilian et al., 2006). It has played a crucial role in the development of agriculture and human societies. Barley is highly adaptable to different environmental conditions, making it a valuable crop in diverse agro-ecological zones. It is used for various purposes, including food, animal feed, and brewing, and continues to be a staple crop in many parts of the world (Dawson et al., 2015).
Nucleotide diversity refers to the variation in DNA sequences among individuals of a species. This genetic diversity is essential for the adaptability and resilience of crops. High nucleotide diversity allows for a greater range of traits that can be selected for breeding, enabling the development of new varieties that can withstand environmental stresses, diseases, and changing climatic conditions. In the context of domestication, however, there is often a reduction in nucleotide diversity due to selective breeding practices that favor specific traits, leading to genetic bottlenecks(Kilian et al., 2006). Understanding and preserving nucleotide diversity is therefore critical for the continued improvement and sustainability of crop species (Haas et al., 2020).
The aim of this study is to review the impact of domestication on barley nucleotide diversity, explore the changes in barley genetic structure during domestication, and the impact of these changes on crop breeding and genetic resource conservation. Through in-depth research on the genetic mechanisms of barley domestication, the genetic resources of barley can be better utilized, providing higher quality seeds and more efficient breeding methods for agricultural production. At the same time, it provides reference and inspiration for the domestication and genetic diversity research of other crops.
1 The History and Background of Domestication of Barley
1.1 Origin and distribution of barley
The origin of barley is closely linked to the Fertile Crescent, where wild progenitors of several key agricultural cereal species, including barley, are endemic. Archaeological evidence suggests that barley was domesticated in this region, particularly in the Jordan Valley. This domestication process led to a reduction in genetic diversity, as evidenced by the comparison of haplotypes between wild barley (Hordeum. vulgare subsp. spontaneum) and domesticated barley (Hordeum vulgare). For instance, domesticated barley lines show a significant reduction in the number of haplotypes and nucleotide diversity compared to their wild counterparts (Kilian et al., 2006).
Further studies have shown that the domesticated gene pools of barley were derived from multiple wild ancestors, indicating a complex domestication process. The distribution of recombination events along barley chromosomes is uneven, with reduced diversity in the pericentromeric regions of both cultivars and landraces compared to wild barley. This pattern suggests that domesticated barley underwent significant genetic bottlenecks, which reduced its genetic diversity (Chen et al., 2020).
1.2 The historical process of domestication of barley
The domestication of barley involved a series of genetic and evolutionary changes that were driven by both natural and human selection. Initially, wild barley exhibited a brittle rachis, which allowed seeds to disperse naturally. However, for early farmers, this trait was undesirable as it made harvesting difficult. Mutations in two adjacent, dominant, and complementary genes led to the development of a non-brittle rachis, which retained grains on the inflorescence at maturity, thus enabling effective harvesting (Pourkheirandish et al., 2015).
Genetic studies have shown that domesticated barley lines exhibit significantly reduced nucleotide diversity compared to their wild counterparts. For instance, the number of haplotypes and average nucleotide diversity were markedly lower in domesticated barley, indicating a loss of genetic variation due to domestication bottlenecks (Kilian et al., 2006). This reduction in diversity was observed across multiple loci, with some loci becoming monomorphic in domesticated lines (Kilian et al., 2006).
Interestingly, the domestication process was not uniform across all regions. Independent selections of germplasm with non-brittle rachis were made in different parts of the Levant, suggesting that barley domestication occurred in multiple locations within the Fertile Crescent (Pourkheirandish et al., 2015). This regional variation in domestication events contributed to the genetic diversity observed in modern barley cultivars.
Furthermore, the domestication of barley also involved changes in the pericentromeric regions of chromosomes, which are typically low in recombination events. These regions showed dramatically reduced diversity in domesticated barley compared to wild barley, indicating that domesticated gene pools were derived from multiple wild ancestors (Figure 1) (Chen et al., 2022). The evolutionary patterns of these regions were shaped by linkage disequilibrium and domestication, highlighting the complex genetic landscape of barley domestication (Chen et al., 2022).
 Figure 1 Signatures of positive selection in barley differentiated by chromosome and zone (Adopted from Chen et al., 2022) Image caption: (a) Selective sweep signal (μ) of barley genomes. Red colours represent genomic regions with μ values above the 95th percentile. The top track shows the chromosome diagrams, with the gradient of blue colours representing zone 1 (light blue), zone 2 (medium blue) and zone 3 (dark blue) regions, and the red bars representing the centromere; (b) Distribution of μ values by chromosome for different barley groups; (c) μ values by zone (data from all seven chromosomes combined) for different barley groups (Adopted from Chen et al., 2022) |
Chen et al. (2022) demonstrated the variation of barley varieties, local varieties, and wild barley on different genomic segments (Figure 1). By analyzing the positive sweep signal, they revealed that specific regions of the genome were strongly selected during domestication. The study found that wild barley displayed strong positive sweep signals in different chromosomal regions, indicating that domestication gene pools may have originated from multiple wild ancestors.
1.3 Genetic structural changes in barley during domestication process
Domestication has led to a significant reduction in genetic diversity in barley. Studies comparing wild and domesticated barley have shown a marked decrease in nucleotide diversity and haplotype number in domesticated lines (Kilian et al., 2006). For instance, the number of haplotypes and average nucleotide diversity (π) were significantly lower in domesticated barley compared to its wild counterpart (Kilian et al., 2006). This reduction in diversity is attributed to bottlenecks during domestication and subsequent breeding processes (Kilian et al., 2006). Additionally, structural variations such as copy number variations (CNVs) have been observed, with higher levels of CNV diversity present in wild barley compared to cultivated forms (Muñoz‐Amatriaín et al., 2013). These CNVs are often associated with genes involved in disease resistance and other agronomically important traits (Muñoz‐Amatriaín et al., 2013).
The domestication process also involved changes in specific genes that contributed to the agronomic traits selected by early farmers. For example, the HvWAK1 gene, which is involved in root proliferation, showed reduced sequence diversity in domesticated barley, suggesting selection for particular cis-regulatory variants that may have indirectly influenced seed size through increased plant vigor (Czajkowska et al., 2019). Furthermore, the evolution of the grain dispersal system in barley involved mutations in two adjacent genes that converted the brittle rachis of wild barley into a tough, non-brittle form, facilitating effective harvesting (Pourkheirandish et al., 2015).
2 Nucleotide Diversity and Research Methods
2.1 Nucleotide diversity and measurement
Nucleotide diversity is a measure of genetic variation within a population. It quantifies the degree of polymorphism at the nucleotide level, providing insights into the genetic health, evolutionary history, and adaptive potential of a species. Nucleotide diversity is typically represented by the symbol π (pi) and is calculated as the average number of nucleotide differences per site between any two DNA sequences chosen randomly from the population.
Nucleotide diversity refers to the variation at the nucleotide level within a population. It is a measure of genetic variation and is crucial for understanding the evolutionary processes and genetic health of a species. Nucleotide diversity is typically quantified using metrics such as the average number of nucleotide differences per site between two DNA sequences chosen randomly from the population. This measure helps in identifying regions of the genome that are under selection and those that are neutral (Russell et al., 2011).
2.2 Development and application of genome sequencing technology
Genomic sequencing technology refers to the technique of sequencing the entire genome of an organism. With the continuous development of sequencing technology, from the first generation sequencing technology to the second and third generation sequencing technology, the throughput, accuracy, and speed of sequencing have been greatly improved, enabling a deeper understanding of the genome structure, function, and evolutionary process of organisms. The application of genome sequencing technology is very extensive, including research on human genetic diseases, animal and plant genes, genomic medicine, drug development, and other fields.
The advent of genome sequencing technologies has revolutionized the study of nucleotide diversity. High-throughput sequencing methods, such as RNA sequencing, allow for the comprehensive analysis of genetic variation across the entire genome. These technologies enable the identification of single nucleotide polymorphisms (SNPs) and other genetic markers at an unprecedented scale and resolution. For instance, deep transcriptome sequencing has been used to explore sequence variations in transcribed sequences of barley, revealing tens of thousands of SNPs and their genome-wide distribution (Takahagi et al., 2016). This approach is particularly useful for species with large and complex genomes, such as barley (Takahagi et al., 2016).
2.3 Detection and analysis of single nucleotide polymorphism (SNP)
Single nucleotide polymorphism (SNP) refers to the genetic marker formed by the variation of a single nucleotide in the genome. This variation is widely present in the human genome, with an average of 1 SNP per 500-1 000 base pairs. SNPs have significant implications in human genetic diseases, differences in drug response, and human evolution.
SNPs are the most common type of genetic variation among individuals of a species. They are single base-pair changes in the DNA sequence and serve as valuable markers for genetic studies. The detection and analysis of SNPs involve sequencing DNA from multiple individuals and comparing the sequences to identify variations. In barley, SNP platforms have been employed to assess the evolution and domestication processes. For example, a study using an oligonucleotide pool assay SNP platform analyzed over 1 000 SNPs in geographically matched samples of landrace and wild barley, providing insights into the genetic differentiation and hybridization events in barley populations (Russell et al., 2011). Another study sequenced alleles at multiple loci in barley, identifying numerous SNPs and using them to construct phylogenetic trees and analyze genetic relationships.
2.4 Application of molecular markers in nucleotide diversity research
Molecular markers refer to DNA sequence variations that can be stably inherited and easily detected. In the study of nucleotide diversity, molecular markers are widely used in population genetic structure analysis, phylogenetic identification, gene mapping, and other aspects. Common molecular markers include restriction fragment length polymorphism (RFLP), randomly amplified polymorphic DNA (RAPD), amplified fragment length polymorphism (AFLP), and SNP. These markers have different characteristics and applicability, and suitable marker types can be selected based on specific research objectives and conditions.
Molecular markers, such as SNPs, are essential tools in nucleotide diversity research. They enable the identification of genetic variations associated with important traits and the study of evolutionary relationships. In barley, molecular markers have been used to investigate the genetic basis of domestication and adaptation. For instance, SNP data have been utilized to differentiate between landrace and wild barley, identify regions of the genome under selection, and suggest possible hybridization events that contribute to the continued adaptation of barley under cultivation (Russell et al., 2011). Additionally, the use of SNP markers has facilitated the study of genome-scale properties of sub-populations in barley, revealing distinct genomic variations between oriental and occidental barley populations (Takahagi et al., 2016).
3 Impact of Domestication on Nucleotide Diversity in Barley
3.1 The impact of domestication on genetic diversity of barley
Domestication has significantly affected the genetic diversity of barley. Research shows that compared with wild barley, the nucleotide diversity of domesticated barley has been significantly reduced. For example, the genetic diversity loss between wild barley and domesticated cultivated barley varies with different chromosomes, and the difference of 5H chromosome is the largest, which is 35.29% (Table 1) (Yan et al., 2015). This reduction in diversity is consistent with the research results of other crops such as wheat and rice, where domestication leads to a significant reduction in genetic variation (Haudry et al., 2007; Zhu et al., 2007).
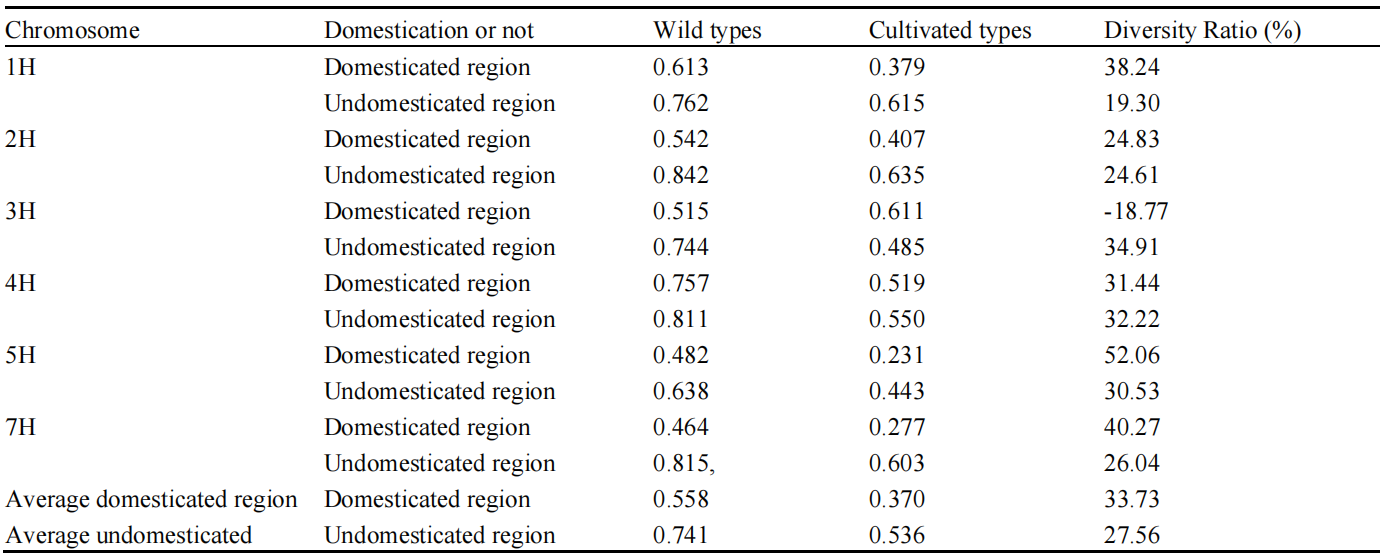 Table 1 Genetic diversity and diversity ratio in domesticated and non-domesticated gene regions of barley chromosomes (Adapted from Yan et al., 2015) Note: diversity ratio = (diversity of wild type - diversity of cultivated type)/diversity of wild typex100% (Adapted from Yan et al., 2015) |
The domestication process often involves genetic bottlenecks, which result in a reduced effective population size and a loss of genetic diversity. In barley, this bottleneck effect is evident, with significant reductions in genetic diversity observed in domesticated regions of the genome (Yan et al., 2015). Similar bottlenecks have been documented in other crops, such as maize and rice, where the founding populations during domestication were relatively small, leading to a severe reduction in genetic diversity (Tenaillon et al., 2004; Zhu et al., 2007).
Genetic drift and selection pressure during domestication have further shaped the genetic landscape of barley. The reduction in effective population size due to bottlenecks increases the impact of genetic drift, altering genotype frequencies and reducing overall diversity (Smýkal et al., 2018). Additionally, selection for agronomically important traits has led to directional selection at specific loci, further reducing genetic diversity in domesticated barley (Tenaillon et al., 2004; Beissinger et al., 2015).
3.2 The impact of domestication on the adaptive genes of barley
Domestication has also influenced the adaptive genes in barley. The process has led to changes in gene regulation and expression, particularly in response to environmental stresses. For example, studies have shown that domestication has resulted in a reduction of sequence diversity in genes and regulatory regions, impacting the expression of adaptive genes under stress conditions (Haas et al., 2020).
Adaptive genes play a crucial role in determining crop yield, quality, and other important traits. The selection of favorable haplotypes around these genes during domestication has created genetic valleys with low diversity, which can affect traits such as grain softness and end-use quality (Haudry et al., 2007). Understanding the correlation between adaptive genes and these traits is essential for improving crop performance and resilience (Haudry et al., 2007).
The knowledge of adaptive genes and their regulation can be applied in crop breeding to enhance desirable traits. For instance, genes regulated in cis are more likely to be expressed consistently in new genetic backgrounds, making them valuable targets for crop improvement using wild relatives (Haas et al., 2020). This approach can help in developing barley varieties with improved stress tolerance and yield.
Haas et al. (2020) compared the transcriptional level differences between unidirectional and bidirectional non coding RNAs using two different statistical methods, limma and binary, under both cold and hot conditions. The study revealed the expression patterns of these genes under different conditions by comparing the differences in cis regulation and possible trans regulation, as well as the data from the control group and the cold treatment group . It was found that cis regulated genes have characteristics such as stability, predictability, and direct effects.
3.3 The impact of domestication on the genome structure of barley
Domestication has led to changes in the genome structure of barley, including alterations in recombination rates and genomic variation. The reduction in genetic diversity due to bottlenecks and selection has impacted the overall genomic landscape, with certain regions showing higher divergence between wild and domesticated forms (Yan et al., 2015).
The domestication process has also resulted in changes in chromosome structure. Comparative studies have shown that domesticated barley exhibits different patterns of genetic diversity across chromosomes, with some regions experiencing more significant changes than others (Yan et al., 2015). These structural changes can influence gene expression and the overall adaptability of the crop.
Copy number variation (CNV) and gene expression are also affected by domestication. The loss of nucleotide diversity can impact regulatory elements, leading to changes in the expression balance of gene isoforms. For example, domesticated sorghum shows less variation in isoform expression balance compared to its wild relatives, suggesting that domestication can lead to more homogenous gene expression patterns (Ranwez et al., 2017).
4 Case Analysis of the Impact of Domestication on Nucleotide Diversity in Barley
4.1 Research cases on domestication of barley at home and abroad
The domestication of barley (Hordeum vulgare) has been a subject of extensive research, revealing significant insights into the genetic and evolutionary consequences of this process. Domestication has led to a marked reduction in nucleotide diversity, a phenomenon observed in various studies.
The study conducted by Kilian et al. (2026) examined the haplotype structure of seven barley genes and compared them with the haplotypes at the same loci of 25 wild forms collected within and outside the Fertile Crescent. The research found a significant reduction in nucleotide diversity in domesticated barley, with the number of haplotypes dropping from 70 in wild forms to 17 in domesticated lines. This reduction was attributed to bottlenecks during domestication and subsequent breeding processes, which significantly decreased genetic diversity at most loci.
The research by Smýkal et al. (2018) on genetic changes during crop domestication has highlighted the broader implications of these processes. Domestication often involves selective sweeps, where favorable haplotypes are retained around selected genes, leading to a genetic valley with extremely low genetic diversity. This phenomenon has been observed in barley, where selection for traits such as seed size and retention has resulted in reduced genetic diversity. The study also noted that genetic bottlenecks during domestication or founding events, as crops moved away from their centers of origin, further altered gene pools, contributing to the reduced nucleotide diversity observed in domesticated barley.
Additionally, the domestication of barley has involved changes in morphological features, such as seed size and inflorescence architecture. The identification of genes like SIX-ROWED SPIKE 1 (VRS1) and INTERMEDIUM-C (INT-C), which are involved in these morphological changes, underscores the genetic modifications that have occurred during domestication. These genes have been linked to variations in lateral spikelet fertility, further illustrating the genetic impact of domestication on barley (Ramsay et al., 2011).
4.2 Nucleotide diversity analysis of specific genes or genomic regions
The impact of domestication on nucleotide diversity can be further understood by analyzing specific genes or genomic regions. For instance, the Rrs2 scald resistance gene region in barley showed more nucleotide diversity in wild barley compared to cultivated barley, with three distinct haplotype groups detected across samples from different countries and regions (Fu, 2012). This indicates that domestication has led to a reduction in genetic diversity in this specific genomic region.
The analysis of single nucleotide polymorphisms (SNPs) in barley conducted by Russell et al. (2011) revealed significant chromosome-level differences in diversity around domestication genes, indicating that certain genomic regions have been more affected by domestication than others. This study also provided evidence that hybridization serves as a mechanism for the continued adaptation of landrace barley under cultivation conditions (Russell et al., 2011).
Moreover, a study on the genetic divergence in domesticated and non-domesticated gene regions of barley chromosomes found that domesticated regions on chromosomes 5H, 1H, and 7H had higher diversity ratios compared to non-domesticated regions (Yan et al., 2015). This indicates that domestication has led to a more pronounced reduction in nucleotide diversity in specific genomic regions associated with domestication traits (Yan et al., 2015).
4.3 Comparative study on the effects of domestication on nucleotide diversity in barley
Domestication has led to significant genetic differentiation between wild and cultivated barley. A study using over 1 000 single nucleotide polymorphisms (SNPs) in geographically matched samples of landrace and wild barley from Jordan and Syria revealed clear genetic differentiation between the two groups, with limited secondary contact (Russell et al., 2011). This differentiation is indicative of the genetic bottleneck that occurred during domestication, which reduced nucleotide diversity in cultivated barley.
The impact of domestication on nucleotide diversity varies across different chromosomal regions. Yan et al. (2015) investigated the genetic divergence in domesticated and non-domesticated gene regions of barley chromosomes and found that chromosome 5H exhibited the highest divergence, with a 35.29% reduction in diversity, followed by chromosomes 3H, 7H, 4H, 2H, and 6H. This study emphasizes that domesticated regions generally show a higher loss of diversity compared to non-domesticated regions, with an average diversity reduction of 33.73% in domesticated regions versus 27.56% in non-domesticated regions.
Domestication has also affected gene regulation and expression in barley. An investigation into the contribution of cis- and trans-acting variants to gene regulation in wild and domesticated barley under cold stress conditions found that most genes have conserved regulation, with a notable absence of trans effects (Haas et al., 2020). This stability in gene regulation suggests that domestication has not drastically altered the regulatory mechanisms in barley, although the overall sequence diversity has been reduced.
Comparative studies with other crops, such as common bean and wheat, provide additional insights into the effects of domestication on nucleotide diversity. For instance, domestication in common bean resulted in a 60% loss of nucleotide diversity and an 18% reduction in gene expression diversity (Bellucci et al., 2014). Similarly, wheat experienced a significant reduction in nucleotide diversity, with bread wheat losing 69% and durum wheat losing 84% of its diversity during domestication. These findings underscore the common trend of reduced genetic diversity following domestication across different crop species.
Despite the reduction in nucleotide diversity, domesticated barley has adapted to a wide range of agricultural environments. Exome sequencing of geographically diverse barley landraces and wild relatives revealed that patterns of genetic variation are strongly shaped by geography, with significant correlations between genetic traits and environmental variables such as temperature and dryness (Russell et al., 2016). This adaptation is facilitated by the extensive sequence variation in flowering-associated genes, which exhibit strong geographical structuring and contribute to regional success.
5 The Significance and Challenges of Domestication on Nucleotide Diversity in Barley
Domestication has played a crucial role in shaping the genetic makeup of barley, one of the oldest cultivated crops. The process of domestication, which began around 10 500 years ago in the Fertile Crescent, involved selecting traits favorable for agriculture, such as increased seed size and non-shattering spikes (Kilian et al., 2006). This selection process, while beneficial for crop yield and ease of harvest, has led to a significant reduction in nucleotide diversity. For instance, domesticated barley shows a marked decrease in haplotype number and nucleotide diversity compared to its wild progenitors (Kilian et al., 2006). This reduction in genetic diversity poses challenges for crop resilience and adaptability, as it limits the genetic pool available for breeding programs aimed at improving disease resistance and environmental stress tolerance (Smýkal et al., 2018).
5.1 Inspiration for crop breeding and genetic improvement
The study of domestication genes in barley provides valuable insights for crop breeding and genetic improvement. Key domestication traits, such as the six-rowed spike, have been linked to specific genetic changes, offering a framework for understanding and manipulating these traits in modern breeding programs (Ramsay et al., 2011). The identification of genes like SIX-ROWED SPIKE 1 (VRS1) and its modifiers, such as INTERMEDIUM-C (INT-C), which is an ortholog of the maize domestication gene TEOSINTE BRANCHED 1, highlights the potential for using genetic knowledge to enhance barley yields and adaptability (Ramsay et al., 2011). Moreover, the discovery of photoperiod-response genes shared between barley and rice suggests that similar genetic networks can be targeted across different crops to improve agricultural productivity.
5.2 Impact on diversity and sustainability of agricultural ecosystems
The reduction in nucleotide diversity due to domestication has significant implications for the sustainability of agricultural ecosystems. Lower genetic diversity in domesticated barley can lead to increased vulnerability to pests, diseases, and changing environmental conditions (Smýkal et al., 2018). This genetic bottleneck effect, observed in both barley and other crops like wheat, underscores the importance of maintaining and utilizing wild relatives and landraces in breeding programs to reintroduce lost genetic variation (Russell et al., 2011). The ongoing adaptation of landrace barley through hybridization with wild types, as evidenced by secondary contact and chromosome-level differences in diversity, suggests that maintaining a diverse genetic pool is crucial for the long-term sustainability of barley cultivation (Russell et al., 2011).
5.3 Challenges and future research directions
One of the primary challenges in barley domestication research is understanding the complex interplay between selection, genetic drift, and gene flow. The significant reduction in genetic diversity observed in domesticated barley necessitates a comprehensive approach to identify and preserve beneficial alleles from wild populations (Kilian et al., 2006, Smýkal et al., 2018). Future research should focus on leveraging advanced molecular technologies, such as genome sequencing and SNP analysis, to map the genetic architecture of domestication traits and identify regions of the genome under selection (Russell et al., 2011, Smýkal et al., 2018). Additionally, exploring the demographic histories of barley domestication through population-level molecular analyses can provide insights into the origins and spread of domesticated barley, informing strategies for genetic conservation and improvement (Smýkal et al., 2018). Addressing these challenges will be critical for enhancing the resilience and productivity of barley in the face of global agricultural demands.
6 Concluding Remarks
This study discusses the impact of domestication on the nucleotide diversity and genetic structure of barley, revealing the loss of genetic diversity in barley during domestication, especially the loss of alleles and the decrease in nucleotide diversity. It further explores the diversity changes in specific gene regions during domestication and analyzes how these changes affect the genetic structure of barley. These findings not only enhance the understanding of the domestication history of barley, but also provide new perspectives for genetic improvement and breeding of barley.
The study of the impact of domestication on barley nucleotide diversity is not only an academic pursuit, but also an important practice in ensuring food security and addressing global challenges. Through in-depth research and interdisciplinary cooperation, it is expected to unlock the genetic potential of barley, cultivate new varieties that are more adaptable to the environment, yield higher, and have better quality, and provide solid food security for the future of humanity.
As mentioned in this study, domestication often accompanies a decrease in genetic diversity and targeted selection of specific genes. However, it is these choices that have gradually adapted barley to different growth environments and human needs. Therefore, future research should focus on the changes of specific genes during domestication and their response to environmental stress, in order to better understand the adaptability of barley.
Acknowledgments
We sincerely thank the peer reviewers for their in-depth analysis and valuable feedback on our manuscript,their evaluations and suggestions provide important perspectives for the improvement of the manuscript.
Conflict of Interest Disclosure
The authors affirm that this research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Beissinger T., Wang L., Crosby K., Durvasula A., Hufford M., and Ross-Ibarra J., 2015, Recent demography drives changes in linked selection across the maize genome, Nature Plants, 2: 16084.
https://doi.org/10.1038/nplants.2016.84
Bellucci E., Bitocchi E., Ferrarini A., Benazzo A., Biagetti E., Klie S., Minio A., Rau D., Rodriguez M., Panziera A., Venturini L., Attene G., Albertini E., Jackson S., Nanni L., Fernie A., Nikoloski Z., Bertorelle G., Delledonne M., and Papa R., 2014, Decreased nucleotide and expression diversity and modified coexpression patterns characterize domestication in the common bean, Plant Cell, 26: 1901-1912.
https://doi.org/10.1105/tpc.114.124040
PMid:24850850 PMCid:PMC4079357
Chen Y., Schreiber M., Bayer M., Dawson I., Hedley P., Lei L., Akhunova A., Liu C., Smith K., Fay J., Muehlbauer G., Steffenson B., Morrell P., Waugh R., and Russell J., 2022, The evolutionary patterns of barley pericentromeric chromosome regions, as shaped by linkage disequilibrium and domestication, The Plant Journal., 111: 1580-1594.
https://doi.org/10.1111/tpj.15908
PMid:35834607 PMCid:PMC9546296
Czajkowska B., Jones G., and Brown T., 2019, Diversity of a wall-associated kinase gene in wild and cultivated barley, PLoS ONE, 14(6): e0218526.
https://doi.org/10.1371/journal.pone.0218526
PMid:31247008 PMCid:PMC6597065
Dawson I., Russell J., Powell W., Steffenson B., Thomas W., and Waugh R., 2015, Barley: a translational model for adaptation to climate change, The New phytologist, 206(3): 913-931.
https://doi.org/10.1111/nph.13266
PMid:25605349
Fu Y., 2012, Population-based resequencing analysis of wild and cultivated barley revealed weak domestication signal of selection and bottleneck in the Rrs2 scald resistance gene region, Genome, 55(2): 93-104.
https://doi.org/10.1139/G11-082
PMid:22272833
Haas M., Himmelbach A., and Mascher M., 2020, The contribution of cis- and trans-acting variants to gene regulation in wild and domesticated barley under cold stress and control conditions, Journal of Experimental Botany, 71: 2573-2584.
https://doi.org/10.1093/jxb/eraa036
Haudry A., Cenci A., Ravel C., Bataillon T., Bataillon T., Brunel D., Poncet C., Hochu I., Poirier S., Santoni S., Glémin S., and David J., 2007, Grinding up wheat: a massive loss of nucleotide diversity since domestication, Molecular Biology and Evolution, 24(7): 1506-1517.
https://doi.org/10.1093/MOLBEV/MSM077
PMid:17443011
Kilian B., Özkan H., Kohl J., Haeseler A., Barale F., Deusch O., Brandolini A., Yucel C., Martin W., and Salamini F., 2006, Haplotype structure at seven barley genes: relevance to gene pool bottlenecks, phylogeny of ear type and site of barley domestication, Molecular Genetics and Genomics, 276: 230-241.
https://doi.org/10.1007/s00438-006-0136-6
PMid:16758198
Muñoz‐Amatriaín M., Eichten S., Wicker T., Richmond T., Mascher M., Steuernagel B., Scholz U., Ariyadasa R., Spannagl M., Nussbaumer T., Mayer K., Taudien S., Platzer M., Jeddeloh J., Springer N., Muehlbauer G., and Stein N., 2013, Distribution, functional impact, and origin mechanisms of copy number variation in the barley genome, Genome Biology, 14: R58.
https://doi.org/10.1186/gb-2013-14-6-r58
PMid:23758725 PMCid:PMC3706897
Pourkheirandish M., Hensel G., Kilian B., Senthil N., Chen G., Sameri M., Azhaguvel P., Sakuma S., Dhanagond S., Sharma R., Mascher M., Himmelbach A., Gottwald S., Nair S., Tagiri A., Yukuhiro F., Nagamura Y., Kanamori H., Matsumoto T., Willcox G., Middleton C., Wicker T., Walther A., Waugh R., Fincher G., Stein N., Kumlehn J., Sato K., and Komatsuda T., 2015,. Evolution of the grain dispersal system in barley, Cell, 162: 527-539.
https://doi.org/10.1016/j.cell.2015.07.002
PMid:26232223
Ramsay L., Comadran J., Druka A., Marshall D., Thomas W., Macaulay M., MacKenzie K., Simpson C., Fuller J., Bonar N., Hayes P., Lundqvist U., Franckowiak J., Close T., Muehlbauer G., and Waugh R., 2011, INTERMEDIUM-C, a modifier of lateral spikelet fertility in barley, is an ortholog of the maize domestication gene TEOSINTE BRANCHED 1, Nature Genetics, 43: 169-172.
https://doi.org/10.1038/ng.745
PMid:21217754
Ranwez V., Serra A., Pot D., and Chantret N., 2017, Domestication reduces alternative splicing expression variations in sorghum, PLoS ONE, 12(9): e0183454.
https://doi.org/10.1371/journal.pone.0183454
PMid:28886042 PMCid:PMC5590825
Russell J., Dawson I., Flavell A., Steffenson B., Weltzien E., Booth A., Ceccarelli S., Grando S., and Waugh R., 2011, Analysis of >1000 single nucleotide polymorphisms in geographically matched samples of landrace and wild barley indicates secondary contact and chromosome-level differences in diversity around domestication genes, The New Phytologist, 191(2): 564-578.
https://doi.org/10.1111/j.1469-8137.2011.03704.x
PMid:21443695
Smýkal P., Nelson M., Berger J., and Wettberg E., 2018, The Impact of Genetic Changes during Crop Domestication, Agronomy, 8(3): 26.
https://doi.org/10.3390/AGRONOMY8030026
Takahagi K., Uehara-Yamaguchi Y., Yoshida T., Sakurai T., Shinozaki K., Mochida K., and Saisho D., 2016, Analysis of single nucleotide polymorphisms based on RNA sequencing data of diverse bio-geographical accessions in barley, Scientific Reports, 6: 33199.
https://doi.org/10.1038/srep33199
PMid:27616653 PMCid:PMC5018957
Tenaillon M., U’Ren J., Tenaillon O., and Gaut B., 2004, Selection versus demography: a multilocus investigation of the domestication process in maize, Molecular Biology and Evolution, 21(7): 1214-1225.
https://doi.org/10.1093/MOLBEV/MSH102
PMid:15014173
Yan S., Sun D., and Sun G., 2015, Genetic Divergence in domesticated and non-domesticated gene regions of barley chromosomes, PLoS ONE, 10(3): e0121106.
https://doi.org/10.1371/journal.pone.0121106
PMid:25812037 PMCid:PMC4374956
Zhu Q., Zheng X., Luo J., Gaut B., and Ge S., 2007, Multilocus analysis of nucleotide variation of Oryza sativa and its wild relatives: severe bottleneck during domestication of rice, Molecular Biology and Evolution, 24(3): 875-888.
https://doi.org/10.1093/MOLBEV/MSM005
.png)
. PDF(540KB)
. FPDF(win)
. FPDF(mac)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Qing Xu
. Renxiang Cai
Related articles
. Barley ( Hordeum vulgare )
. Domestication of barley
. Nucleotide diversity
. Genetic diversity
. Targeted breeding
Tools
. Email to a friend
. Post a comment


