Research Article
Impact of Osmotic Stress on Biochemical and Physiological Parameters in Zea mays L. cv. Ganga Safed-2 Genotype 
 Author
Author  Correspondence author
Correspondence author
Maize Genomics and Genetics, 2016, Vol. 7, No. 1 doi: 10.5376/mgg.2016.07.0001
Received: 31 Dec., 2015 Accepted: 20 Jun., 2016 Published: 13 Jul., 2016
Swati T., and Meeta J., 2016, Impact of Osmotic Stress on Biochemical and Physiological Parameters in Zea mays L. cv. Ganga Safed-2 Genotype, Maize Genomics and Genetics, 7(01): 1-14 (doi: 10.5376/mgg.2016.07.0001)
Treatment of maize leaf segments from etiolated seedlings with different concentrations of sorbitol during greening decreased the fresh weight, turgid weight and dry weight significantly, being indicated by higher R squared values obtained on correlation analysis. Concentration dependent decrease in protein and RNA contents were found with increasing concentration of sorbitol. Osmotic stress affects total DNA content. Incubation of leaf segments with sorbitol decreased the total chlorophylls and carotenoids in a concentration dependent manner. Reduction in chlorophylls was to a higher extent than carotenoids with highly significant R squared value. Further, chlorophyll a, chlorophyll b also decreased with increasing concentrations of sorbitol. It seems that water and metabolic status of leaf segments is less affected by osmotic stress, which is apparent from marginal decreasing effect of sorbitol on RWC, protein and RNA content. Strong correlation between sorbitol concentration and various parameters measured in dark grown maize leaf segments, with the R squared values being 0.958 with fresh weight, 0.968 with turgid weight, 0.969 with dry weight, 0.968 with total chlorophylls, 0.909 with carotenoids suggest that sorbitol induced osmotic stress has a prominent inhibitory effect on growth and chlorophyll biosynthesis in dark grown maize leaf segments.
1 Introduction
Water deficit is a serious environmental problem that causes osmotic stress and hence reduction in plant growth and crop productivity worldwide, reducing average yields for most major crop plants by more than 50%. Osmotic stress results into dehydration which in turn causes a multitude of changes in molecular, biochemical and physiological phenomenon, thereby, affecting plant growth and development (Zhu, 2002). One of the common adverse effects of water stress on crop plant is the reduction in fresh and dry biomass production (Farooq et al., 2009). Due to low turgor pressure, cell expansion and cell growth are also suppressed under water stress (Shao et al., 2008). Inhibition of leaf growth is a primary whole plant response to water stress which has been reported in maize, barley and rice seedlings (Lu and Neumann, 1998). When water deficit becomes too intense or prolonged, plants can wilt, cells can undergo shrinkage and this may lead to mechanical constraint on cellular membranes. This in turn impairs the functioning of ions and transporters as well as membrane associated enzymes (Bowler et al., 1992). Because of decline in cellular volume, cellular content becomes viscous, which increase the probability of protein-protein interaction leading to their aggregation and denaturation (Mahajan and Tuteja, 2005). Increased concentration of solutes may also exceed toxic levels, which may be deleterious for the functioning of some of the enzymes including the enzymes required for photosynthetic machinery (Hoekstra et al., 2001). The suppressing effect of water deficit on photosynthetic efficiency may be due to closing of stomata, which limits CO2 diffusion into the leaf (Lawlor and Cornic, 2002; Demirevska et al., 2010; Rapacz et al., 2010).
Photosynthesis, the most fundamental and intricate physiological process in all green plants, is also severely affected in all its phases by such stresses. Since the mechanism of photosynthesis involves various components, including photosynthetic pigments and photosystems, the electron transport system, and CO2 reduction pathways, any damage at any level caused by a stress may reduce the overall photosynthetic capacity of a green plant (Ashraf and Harris, 2013). Water deficit condition, hamper the process of photosynthesis in plants by altering the ultra structure of the organelles and concentration of various pigments and metabolites including enzymes involved in this process as well as stomatal regulation. Reduction in chlorophyll level by water stress (Albert and Thornber, 1977; Tomati et al., 1978) and irrigation deficit (Moaveni, 2011) has been shown in maize plant. Oxidative stress is another serious consequence of water deficit in plants with excessive generation of reactive oxygen species (ROS), such as, superoxide, hydroxyl radicals, H2O2 and singlet oxygen (Mittler, 2002). The potential sites for generation of ROS in plants are chloroplast, mitochondria and peroxisomes (Ashraf et al., 1994).
Sorbitol is a low molecular weight alditol which is being used to impose osmotic stress on leaf slices, protoplast or chloroplast. It is most frequently found polyols in plants. The objective of the present study was to analyze the effect of osmotic stress induced by sorbitol on growth and biochemical characteristics in dark grown maize leaves.
2 Results and Discussion
2.1 Sorbitol effect on fresh weight, turgid weight, dry weight and RWC in excised etiolated maize leaf segments during greening
Supply of varying concentration of sorbitol (0.2 M to 1.0 M) to the etiolated maize leaf segments during greening decreased the fresh weight, turgid weight and dry weight gradually with increasing concentration (Table 1). Decrease in dry weight was more substantial at higher concentrations of sorbitol than fresh weight and turgid weight. Marginal reduction in relative water content was observed at all the concentrations of sorbitol (Table 1). Correlation analysis between sorbitol concentrations and these parameters was performed by using Microsoft excel chart type X-Y scatter, the R squared values obtained were highly significant, being 0.958 for fresh weight, 0.968 for turgid weight and 0.969 for dry weight respectively. (Figure 1a; Figure 1b; Figure 1c).
|
Table 1 Effect of sorbitol on fresh weight, turgid weight, dry weight and RWC in excised etiolated maize leaf segments during greening Note: Leaf segments from dark grown maize seedlings were treated with varying concentration of sorbitol in continuous light for 24 h at 25±3°C; values relative to control are given in parentheses; level of significance: ‘p’ values<0.05*, <0.01**, <0.001*** compared with control |
|
Figure 1a Correlation analysis of sorbitol concentration and fresh weight |
|
Figure 1b Correlation analysis of sorbitol concentration and turgid weight |
|
Figure 1c Correlation analysis of sorbitol concentration and dry weight |
2.2 Sorbitol effect on total protein, total RNA and DNA content in excised etiolated maize leaf segments during greening
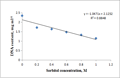
When etiolated maize leaf segments were treated with different concentrations of sorbitol during greening, the protein as well as RNA content decreased from 0.2 to 1.0 M sorbitol (Table 2). The decrease in RNA content at each concentration of sorbitol was less substantial than the protein content (Table 2). DNA content also decreased in a concentration dependent manner in sorbitol treated leaf tissue (Figure 3a). The R squared values obtained from correlation analysis were 0.980 with protein (Figure 2a), 0.991 with RNA (Figure 2b) and 0.884 with DNA content (Figure 3b).
|
Table 2 Effect of sorbitol on total protein and total RNA in excised etiolated maize leaf segments during greening Note: Leaf segments from dark grown maize seedlings were treated with varying concentration of sorbitol in continuous light for 24 h at 25±3 °C; values relative to control are given in parentheses; level of significance: ‘p’ values <0.05*, <0.01**, <0.001***compared with control |
|
Figure 2a Correlation analysis of sorbitol concentration and total protein |
|
Figure 2b Correlation analysis of sorbitol concentration and total RNA |
|
Figure 3a Effect of sorbitol on DNA content in excised etiolated maize leaf segments during greening |
|
Figure 3b Correlation analysis of sorbitol concentration and DNA content |
2.3 Sorbitol effect on total chlorophylls and carotenoids in excised etiolated maize leaf segments during greening
Supply of 0.2 M to 1.0 M sorbitol to etiolated maize leaf segments during greening decreased the total chlorophyll content gradually in a concentration dependent manner. The carotenoid content also decreased from 0.4 M to 1.0 M sorbitol; however it increased at 0.2 M concentration (Table 3). Decrease in chlorophyll content was more prominent than carotenoids (Table 3). The R squared values obtained from correlation analysis between sorbitol treatment and these pigments were highly significant, being 0.969 with total chlorophylls and 0.794 with carotenoid (Figure 4a; Figure 4b).
|
Table 3 Effect of sorbitol on total chlorophylls and carotenoids in excised etiolated maize leaf segments during greening Note: Leaf segments from dark grown maize seedlings were treated with varying concentration of sorbitol in continuous light for 24 h at 25± 3°C; values relative to control are given in parentheses; level of significance: ‘p’ values<0.05 *, <0.01 **, <0.001***compared with control |
|
Figure 4a Correlation analysis of sorbitol concentration and total chlorophylls |
|
Figure 4b Correlation analysis of sorbitol concentration and carotenoids |
2.4 Sorbitol effect on chlorophyll a, chlorophyll b and chlorophyll a/b ratio in excised etiolated maize leaf segments during greening
Treatment of maize leaf segments from etiolated seedlings with different concentrations of sorbitol during greening decreased the chlorophyll a content in a concentration dependent manner except at 0.2 M where it was increased. The chlorophyll b content also decreased with increasing concentration of sorbitol (Table 4). Due to decrease in chlorophyll b content to a greater extent than chlorophyll a, the chlorophyll a/b ratio was higher at all the concentrations of sorbitol (Table 4).
|
Table 4 Effect of sorbitol on chlorophyll a, chlorophyll b and chlorophyll a/b ratio in excised etiolated maize leaf segments during greening Note: Leaf segments from dark grown maize seedlings were treated with varying concentration of sorbitol in continuous light for 24 h at 25±3°C; values relative to control are given in parentheses; level of significance: ‘p’ values <0.05*, <0.01**, <0.001*** compared with control |
2.5 Sorbitol effect on proline content in excised etiolated maize leaf segments during greening
Treatment with sorbitol increased the proline content in a concentration dependent manner. There was 1.5 fold increases at 1.0 M sorbitol (Figure 5a). The R squared values for sorbitol concentration with proline content were 0.961 (Figure 5b).
|
Figure 5a Effect of sorbitol on proline content in excised etiolated maize leaf segments during greening |
|
Figure 5b Correlation analysis of sorbitol concentration and proline content |
Plants are subjected to several harsh environmental stresses that adversely affect growth, metabolism and yield. Amongst these, osmotic stress is a major abiotic factor that limits the agricultural crop’s productivity. As the stress conditions in plant tissue rise, different mechanisms are used by plants to manage the stress before the sudden onset of chronic imbalance. However, plants being sessile organisms, have limited mechanisms for stress avoidance. Chlorophyll content reflects the overall status of growth and photosynthetic productivity of plants.
Maintenance of plant water status is a fundamental phenomenon for normal growth of plants under stressful environment. Disturbances in water balance leads to impaired functioning, hence, reduced plant growth. A common adverse effect of water stress on crop plants is the reduction in fresh and dry biomass production (Farooq et al., 2009). Decrease in fresh weight and dry weight has been reported in pigmented O. sativa seedlings, Malus sylvestris and Cydonia oblonga rootstock under water deficit conditions (Chutipaji et al., 2012; Bolat et al., 2014). Similarly, marked reduction in fresh and dry masses was noted in two maize cultivars under salt stress conditions (Hussain et al., 2013). Plant growth is also affected, when leaf water balance is changed by altering evaporative demand. Transitory changes in fresh weight of leaf lettuce could be ascribed to the changes in tranpiratory rate (Oda and Tsuji, 1992). In the present study, concentration dependent decline in fresh weight, turgid weight and dry weight is observed during greening of dark grown maize leaf segments treated with different concentration of sorbitol (Table 1). Strong correlation between sorbitol treatment and these parameters emphasize a significant negative effect of osmotic stress induced by sorbitol on biomass. RWC may be attributed to differences in the ability of the variation to absorb more water from the soil and/or the ability to control water loss through stomata. Thus, RWC parameter is a measure of water status and can be used to select high yielding genotypes that maintain cell turgor under water stress environment to give relative high yield (Bayoumi et al., 2014). Marginal reduction in RWC is observed in maize leaf segments treated with sorbitol (Table 1).
To analyze the effect of sorbitol stress on overall metabolic activities, total RNA and protein content were measured in the leaf segments. Concentration dependent reduction in both the parameters was found with increasing concentration of sorbitol (Table 2), however, decline in protein content was more prominent than the RNA content. Significant decrease of these parameters indicates the disturbances in the metabolic status of leaf tissue. Correlation analysis between sorbitol concentrations and these parameters yielded significant R squared value, being 0.980 with protein and 0.991 with RNA (Figure 2a; Figure 2b). Decrease in protein content has been reported in O. sativa leaves treated with sorbitol (Hsu and Kao, 2003). Reduction in protein synthesis at both transcriptional and post-transcriptional levels has been demonstrated in water stressed Triticum aestivum leaves (He et al., 1999). Further, they also reported decrease in RNA synthesis with increased water stress and suggested the up regulation of chloroplast RNAase as one of the possible reasons for the degradation of RNA. Furthermore, many researchers showed that the amount of ribosome and the proportion of polyribosome decreased remarkably during water stress (Scott et al., 1979; Mason et al., 1988). Substantial reduction in DNA content in leaf segments treated with varying concentration of sorbitol has also been observed in the present study with R squared value of 0.884 with DNA on correlation analysis (Figure 3a; Figure 3b). This may be due to either direct damaging effect of sorbitol on DNA or indirectly by the free radicals generated in the stressed leaf tissue because of oxidative damage. Chlorophyll formation during irradiation of dark grown plants is influenced by external and internal factors, such as, light quality, temperature, nutrition, leaf water potential and leaf age (Virgin, 2014; Bengston et al., 1978; Le et al., 2007). Photosynthetic pigments are important to plants mainly for harvesting light and generation of reducing powers and energy. Hence, loss of chlorophyll contents under water stress is considered a main cause of inactivation of photosynthesis. Carotenoids have additional roles and partially help the plants to withstand adverse effects of drought. Reduction in chlorophyll content due to water deficit/ drought has been reported in Gossypium. Hirsutum L. (Massaci et al., 2008) and Helianthus annuus L. (Kiani et al., 2008). Significant degradation of chlorophyll pigments due to salt stress has been observed in different varieties of rice (Jamil et al., 2012). In the present study, results reveal a concentration dependent decrease in total chlorophylls and carotenoids in etiolated maize leaf segments during greening treated with increasing concentration of sorbitol (Table 3). Decrease in chlorophyll content was more prominent than carotenoids (Table 3). Sorbitol treatment decreased the chlorophyll a and chlorophyll b content with the decrease in chlorophyll b being more substantial than chlorophyll a and hence increases in chlorophyll a/b ratio. The increase in chlorophyll a/b ratio indicates that the light-dependent phase of photosynthesis works in a narrower range of visible spectrum and ultimately impairs light use efficiency of plant and lower carbon assimilation capability (Table 3). The R squared values obtained from correlation analysis between sorbitol treatment and total chlorophyll was highly significant being 0.969 and 0.794 with carotenoids (Figure 4a; Figure 4b).
Many plants and other organisms cope with osmotic stress by synthesizing and accumulating some compatible solutes, which are termed as osmoprotectant or osmolytes. These include ions, such as, K+, Na+ and Cl- or organic solutes like proline and other amino acids, polyamines and glycine betanine (Tamura et al., 2003). Other osmolytes that are produced in response to stress include sucrose, polyols, sugar alcohols and oligosaccharides. Osmolytes play a major role in osmotic adjustment and also protects the cells by scavenging ROS (Pinhero et al., 2001). In my study, increased level of proline was observed with increasing osmotic stress induced by sorbitol. Multiple functions have been proposed for proline accumulation under stress conditions, eg.- membrane stabilization, maintenance of protein conformation, protection of biomoleceules, such as, lipids, DNA and proteins, radical detoxification etc. (Smirniff and Cumbes, 1989; Smirniff and Stewart, 1985; Sampras et al., 1995). Further, proline accumulation may occur due to activation of its biosynthesis or inactivation of degradation. Enhancement in free proline concomitant with an increase in protease activity has been indicated in O. sativa plants stressed with PEG- 6000 and mannitol (Pandey et al., 2004). Thus, in my investigation also, it is possible that sorbitol treatment may be either increasing protease activity, as the protein content also decreases with increasing osmotic stress (Table 2).
3 Materials and Methods
Sterilized seeds of Zea mays L.cv. Ganga safed-2 were raised in continuous darkness for 7-8 d at 25±3ºC. They were watered with half strength Hoagland solution containing 5 mM ammonium nitrate. For analysis of biochemical parameters, excised segments of primary leaves were treated with different concentrations of sorbitol (0.0-1.0 M) in continuous light supplied with fluorescent tubes for 24 h at 25±3ºC. Treated leaf segments were thoroughly washed with distilled water prior to analysis.
Relative water content was measured by the method of Barr and Weatherly (1962). About 200 mg of excised leaf segments were floated on desired solutions for 24 h at 25±3ºC in continuous light. After treatment the segments were blotted and fresh weight was measured. The dry weight of the leaf segments was determined after drying for 24 h at 80ºC. To obtain the turgid weight, the leaf segments were refloated on distilled water for 4 h in continuous light. The RWC was calculated by using the following equation:
RWC (%)=(FW-DW)/(TW-DW)*100
FW is the fresh weight of the leaf segments; DW is the dry weight and TW is the turgid weight of the leaf segments.
Protein content was estimated with Folin Ciocalteau reagent according to the method of Lowry et al. (1951). Total RNA was extracted and estimated by the method of Webb and Levy (1958) using Orcinol reagent. DNA was extracted from leaves by CTAB method as described by Doyle and Doyle (1990).
The proline content was estimated spectrophotometrically by the method described by Bates et al. (1973).
For the estimation of pigment content, leaf tissue was extracted with 80% acetone in cold. The extract was centrifuged and the absorbance of clear supernatant was measured at 646, 663 and 470 nm. The chl a, chl b and carotenoid contents were calculated using equation of Lichtenthaler and Welburn (1983).
Chlorophyll a (µg/mL)=12.21(A663)-2.81(A646)
Chlorophyll b (µg/mL)=20.13(A646)-5.03(A663)
Carotenoids (µg/mL)=[1000(A470)-3.27(Chl a)-104(Chl b)]/229
4 Conclusion
In the present study the results revealed that the dark grown maize leaf segments exhibit high degree of stress due to sorbitol treatment and it affects the overall growth and biochemical parameters governing the metabolic activities of the leaf tissue. Marginal reductions in RWC of sorbitol treated leaf segments indicate tolerance towards osmotic stress. Further, sorbitol induced osmotic stress has an inhibitory effect on total chlorophylls, carotenoids content. Proline content increased with increasing osmotic stress induced by sorbitol.
Acknowledgement
Authors express thanks for financial support of National Medicinal Plant Board, New Delhi and World Noni Research Foundation, Chennai and the director ICAR-CIARI, Port Blair for laboratory facilities.
Zhu J.K., 2002, Salt and drought stress signal transduction in plants, Annu Rev Plant Biol., 53: 247-273
http://dx.doi.org/10.1146/annurev.arplant.53.091401.143329
PMid:12221975 PMCid:PMC3128348
Farooq M., Wahid A., Kobayashi N., Fujita D., and Basra S.M.A., 2009, Plant drought stress: effects, mechanisms and management, Agron Sustain Dev., 29: 185-212
http://dx.doi.org/10.1051/agro:2008021
Shao H.B., Chu L.Y., Shao M.A., Abdul Jaleel C., and Hong-Mei M., 2008, Higher plant antioxidants and redox signaling under environmental stresses, Comp Rend Biol., 331: 433-441
http://dx.doi.org/10.1016/j.crvi.2008.03.011
PMid:18510996
Lu Z., and Neumann P.M., 1998, Water-stressed maize, barley and rice seedlings show species diversity in mechanisms of leaf growth inhibition , Journal of Experimental Botany, 49: 1945-1952
http://dx.doi.org/10.1093/jxb/49.329.1945
Bowler C., Montagu M.V., and Inze D., 1992, Superoxide dismutase and stress tolerance, Ann. Rev. Plant Physiol. Plant Mol. Biol., 43: 83-116
http://dx.doi.org/10.1146/annurev.pp.43.060192.000503
Mahajan S., and Tuteja N., 2005, Cold, salinity and drought stresses: An overview. Archives of Biochemistry and Biophysics, 444: 139-158
http://dx.doi.org/10.1016/j.abb.2005.10.018
PMid:16309626
Hoekstra F.A., Golovina E.A., and Buitink J., 2001, Mechanisms of plant desiccation tolerance, Trends in Plant Science, 6 (9): 431-438
http://dx.doi.org/10.1016/S1360-1385(01)02052-0
Lawlor D.W., and Cornic G., 2002, Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants, Plant Cell Environ, 25: 275-294
http://dx.doi.org/10.1046/j.0016-8025.2001.00814.x
PMid:11841670
Demirevska K.L., Simova-Stoilova I., Fedina K., Georgieva K., and Kunert K., 2010, Response of Oryzacystatin I transformed tobacco plants to drought, heat and light stress, J Agron Crop Sci., 196: 90-99
http://dx.doi.org/10.1111/j.1439-037X.2009.00396.x
Rapacz M., Koscielniak J., Jurczyk B., Adamska A., and Wojcik M., 2010, Different patterns of physiological and molecular response to drought in seedlings of malt and feed type barleys (Hordeum vulgare), J Agron Crop Sci., 196: 9-19
http://dx.doi.org/10.1111/j.1439-037X.2009.00389.x
Ashraf M., and Harris P.J.C., 2013, Photosynthesis under stressful environments: An overview, Photosynthetica, 51(2): 163-190
http://dx.doi.org/10.1007/s11099-013-0021-6
Albert R.S., and Thornber J.P., 1977, Water stress effect on the content and organization of chlorophyll in mesophyll and bundle sheath chloroplast of maize, Plant Physiol., 59: 351-353
http://dx.doi.org/10.1104/pp.59.3.351
Tomati U., Veri G., and Galli E., 1978, Effect of water status on photosynthesis and nitrate reductase activity in maize plants. Review in Agronomy, 12: 119-122
Moaveni P., 2011, Effect of water deficit stress on some physiological traits of wheat (Triticum aestivum), Agricultural Science Research Journal, 1(3): 64-68
Mittler R., 2002, Oxidative stress, antioxidants and stress tolerance, Trends Plant Sci., 7: 405-410
http://dx.doi.org/10.1016/S1360-1385(02)02312-9
Ashraf M.Y., Azmi A.R., Khan A.H., and Ala S.A., 1994, Effect of water stress on total phenols, peroxidase activity and chlorophyll content in wheat (Triticum aestivum L.) genotypes under soil water deficits, Acta Physiol Plant, 16: 185-191
Barr H.D., and Weatherely P.E., 1962, A re-examination of the relative turgidity technique for estimating water deficits in leaves, Australian Journal of Biological Sciences, 15: 413-428
http://dx.doi.org/10.1071/BI9620413
Lowry O.H., Rosebrough N.J., Farr A.C., and Randall R.J., 1951, Protein measurement with the Folin-Phenol reagent, J Biol Chem.,193(1): 265-275
Webb J.M., and Levy H.B., 1958, New developments in chemical determination of nucleic acid, Inglick D. editor, Methods of Biochemical Analysis, 6: 1-30
http://dx.doi.org/10.1002/9780470110225.ch1
PMid:13577420
Doyle J.J., and Doyle J.L., 1990, A rapid DNA isolation procedure for small quantities of fresh leaf tissue, Phytochemical Bulleti., 19: 11-15
Bates L.S., Waldren R. P. and Teari D., 1973, Rapid determination of free proline for water stress studies, Plant Soil., 39: 205-207
http://dx.doi.org/10.1007/BF00018060
Lichtenthaler H.K., and Welburn A.R., 1983, Determination of total carotenoids and chlorophylls a and b of extract in different solvents, Biochem Soc Trans., 11: 591-592
http://dx.doi.org/10.1042/bst0110591
Farooq M., Wahid A., Kobayashi N., Fujita D., and Basra S.M.A., 2009, Plant drought stress: effects, mechanisms and management, Agron. Sustain. Dev., 29: 185-212
http://dx.doi.org/10.1051/agro:2008021
Chutipaijit S., Cha-um S., and Sompornpailin K., 2012, An evaluation of water deficit tolerance screening in pigmented indica rice genotypes, Pak J Bot., 44(1): 65-72
Bolat I., Dikilitas M., Ercisli S., Ikinci A. and Tonkaz T., 2014, The Effect of Water Stress on Some Morphological, Physiological, and Biochemical Characteristics and Bud Success on Apple and Quince Rootstocks Hindawi Publishing Corporation, The Scientific World Journal Volume Article ID 769732: 1-8
http://dx.doi.org/10.1155/2014/769732
http://dx.doi.org/10.1155/2014/563181
PMid:25101318 PMCid:PMC4101936
Hussain I., Ashraf M.A., Anwar F., Rasheed R., Niaz M., and Wahid A., 2013, Biochemical characterization of maize (Zea mays L.) for salt tolerance, Plant Biosystems., 148(5): 1016-1026
Oda M., and Tsuji K., 1992, Monitoring fresh weight of leaf lettuce (Lactuca sativa), 2: Effects of light, air temperature, relative humidity and wind velocity, JARQ, 26(1): 19-25
Bayoumi T.Y., Eid M.H., and Metwali E.M., 2014, Application of physiological and biochemical indices as a screening technique for drought tolerance in wheat genotypes, Afri. J. Biotech., 7: 2341-2352
He J.X., An L.Z., Lin H.H., and Liang H.G., 1999, Evidence for transcriptional and post-transcriptional control of protein synthesis in water-stressed wheat leaves: a quantitative analysis of messenger and ribosomal RNA, Journal of Plant Physiology, 155: 63-69
http://dx.doi.org/10.1016/S0176-1617(99)80141-2
Scott N.R., Munns R., Barlow E.W.R, 1979, Polyribosome content in young and aged wheat leaves subjected to drought, Journal of Experimental Botany, 30: 905-911
http://dx.doi.org/10.1093/jxb/30.5.905
Mason H.S., Mullet J.E., and Boyer J.S., 1988, Polysomes, messenger RNA, and growth in soybean stems during development and water stress, Plant Physiology, 86: 725-733
http://dx.doi.org/10.1104/pp.86.3.725
PMid:16665977 PMCid:PMC1054559
Virgin H.I., 2014, Chlorophyll formation and water deficit, Physiologia Plantarum, 18: 994-1000
http://dx.doi.org/10.1111/j.1399-3054.1965.tb06995.x
Bengtson C., Klockare B., Klockare R., Larsson S., and Sundqvist C., 1978, The after-effect of water stress on chlorophyll formation during greening and the levels of abscisic acid and proline in dark-grown wheat seedlings, Physiologia Plantarum, 43: 205-212
http://dx.doi.org/10.1111/j.1399-3054.1978.tb02565.x
Le Lay P., Boddi B., Kovacevic D., Juneau P., Dewez D., Jain M., and Raghavendra A.S., 2007, Effect of osmotic stress on production of hydro peroxide in pea (P. sativum) leaves and its relationship to shrinkage, Jour. Pl. Sci. Res., 23(1-2): 87-90
Massacci A., Nabiev S.M., Pietrosanti L., Nematov S.K., Chernikova T.N., Thor K., and Leipner J., 2008, Response of the photosynthetic apparatus of cotton (Gossypium hirsutum) to the onset of drought stress under field conditions studied by gas-exchange analysis and chlorophyll fluorescence imaging, Plant Physiol Biochem., 46: 189-195
http://dx.doi.org/10.1016/j.plaphy.2007.10.006
Kiani S.P., Maury P., Sarrafi A., and Grieu P., 2008, QTL analysis of chlorophyll fluorescence parameters in sunflower (Helianthus annuus L.) under well-watered and water-stressed conditions, Plant Sci., 175: 565-573
http://dx.doi.org/10.1016/j.plantsci.2008.06.002
Jamil M., Bashir S., Anwar S., Bibi S., Bangash A., Ullah F., and Rha E.S, 2012, Effect of salinity on physiological and biochemical characteristics of different varieties of rice, Pak. J. Bot., 44: 7-13
Tamura T., Hara K., Yamaguchi Y., Koizumi N., and Sano H., 2003, Osmotic stress tolerance of transgenic tobacco expressing a gene encoding a membrane-located receptor-like protein from tobacco plants, Plant Physiol., 131: 454-462
http://dx.doi.org/10.1104/pp.102.011007
Pinhero R.G., Rao M.V., Palyath G., Murr D.P., and Fletcher R.A., 2001, Changes in the activities of antioxidant enzymes and their relationship to genetic and paclobutrazol-induced chilling tolerance of maize seedlings, Plant Physiol., 114: 695-704
Smirnoff N., and Cumbes Q.J., 1989, Hydroxyl radical scavenging activity of compatible solutes, Phytochemistry, 28: 1057-1060
http://dx.doi.org/10.1016/0031-9422(89)80182-7
Smirnoff N., and Stewart G.R., 1985, Stress metabolites and their role in coastal plants, Vegetation, 62: 273-278
http://dx.doi.org/10.1007/BF00044753
Sampras Y., Bressan R.A., Csonka L.N., Garcia Rio M.G., Paino D., Urgo M., and Rhodes D., 1995, Proline accumulation during drought and salinity. In: Smirnoff N. (ed.). Environment and Plant Metabolism Flexibility and Acclimation. Bios Scientific publishers, Oxford, UK, pp.161-187
Pandey R., Agarwal R.M., Jeevratnam K., and Sharma G.L., 2004, Osmotic stress-induced alterations in rice (Oryza sativa L.), Biologia Plantarum, 42: 79-87
. PDF(0KB)
. HTML
Associated material
. Readers' comments
Other articles by authors
. Swati Tiwary
. Meeta Jain
Related articles
. Zea mays L.
. Chlorophyll
. Osmotic stress
. Sorbitol
Tools
. Email to a friend
. Post a comment
.png)



.png)



.png)

.png)
.png)




